104981
6-Bromopurine
98%
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C5H3BrN4
Numéro CAS:
Poids moléculaire :
199.01
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
98%
Forme
solid
Pf
>300 °C (lit.)
Groupe fonctionnel
bromo
Chaîne SMILES
Brc1ncnc2nc[nH]c12
InChI
1S/C5H3BrN4/c6-4-3-5(9-1-7-3)10-2-8-4/h1-2H,(H,7,8,9,10)
Clé InChI
CTGFGRDVWBZYNB-UHFFFAOYSA-N
Catégories apparentées
Description générale
6-Bromopurine enhances the carcinostatic activity of azaserine in a test system employing ascites cell forms of sarcoma 180 and Ehrlich carcinoma in vivo. 6-bromopurine nucleosides are excellent substrates for substitution reactions with N-, O-, and S-containing nucleophiles in polar solvents.
Application
6-Bromopurine was used in the synthesis of 6-halopurine alkynes and corresponding triazole derivatives. 6-Bromopurine was used in the synthesis and chemical characterization of 2,3,4,5-tetrahydro-1,5-benzoxazepines-3-ol.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Comparison of some biologgical and biochemical properties of 6-bromopurine and 6-iodopurine.
A C SARTORELLI et al.
Biochemical pharmacology, 11, 1017-1024 (1962-11-01)
E A Véliz et al.
The Journal of organic chemistry, 66(25), 8592-8598 (2001-12-12)
Surprisingly facile direct substitution reactions with acetyl-protected 6-bromopurine nucleosides are described. Included in the series of bromonucleosides studied is the guanosine derivative N(2)-2',3',5'-tetraacetyl-6-bromopurine ribonucleoside, the synthesis of which is reported here for the first time. Brominated nucleosides had not previously
Synthesis, unambiguous chemical characterization, and reactivity of 2, 3, 4, 5-tetrahydro-1, 5-benzoxazepines-3-ol.
Garcia-Rubino ME, et al.
Royal Society of Chemistry Advances, 2(33), 12631-12635 (2012)
Eva Galante et al.
Molecules (Basel, Switzerland), 18(5), 5335-5347 (2013-05-15)
2-[¹⁸F]Fluoroethyl azide ([¹⁸F]FEA) can readily be obtained by nucleophilic substitution of 2-azidoethyl-4-toluenesulfonate with [¹⁸F]fluoride (half-life 110 min), and has become widely used as a reagent for 'click' labeling of PET tracers. However, distillation of [18F]FEA is typically required, which is
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique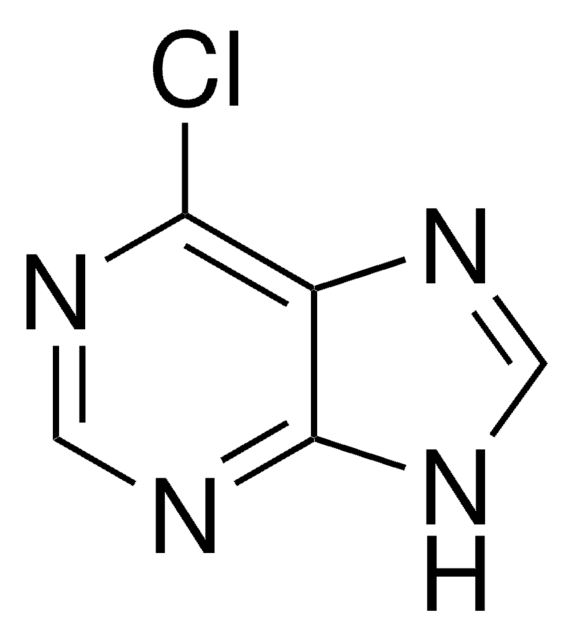
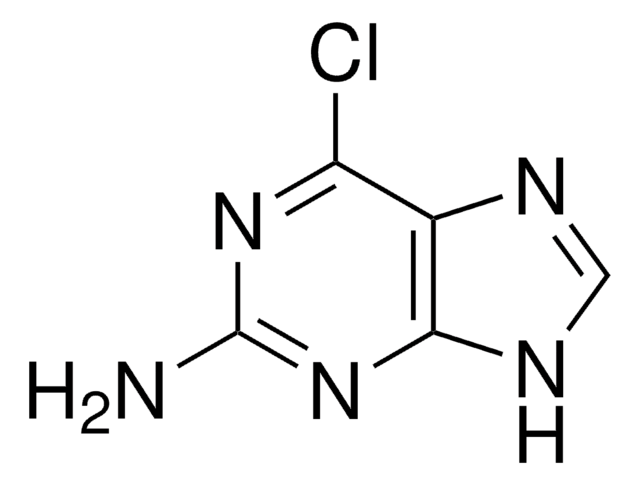
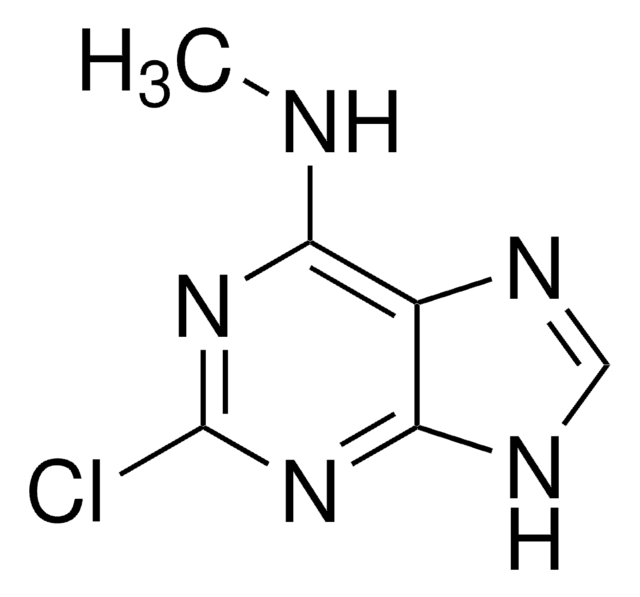
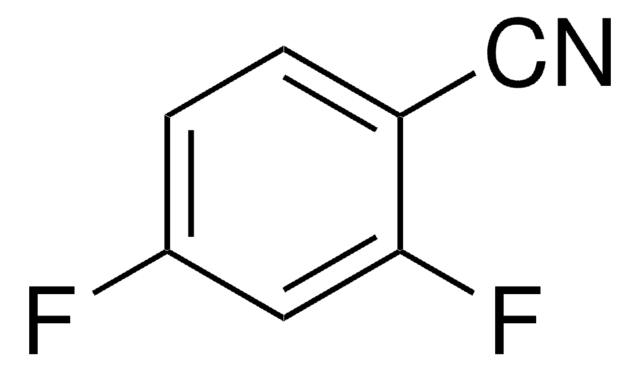
![2,4-Dichloro-7H-pyrrolo[2,3-d]pyrimidine-7-carboxylic acid tert-butyl ester AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/315/036/b807f57a-439c-4114-b92d-077d429f82f3/640/b807f57a-439c-4114-b92d-077d429f82f3.png)
