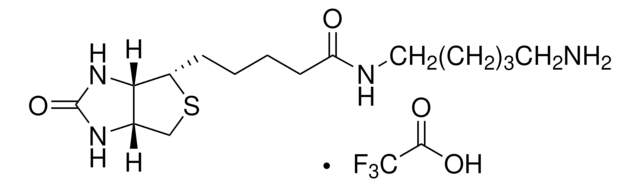
914134
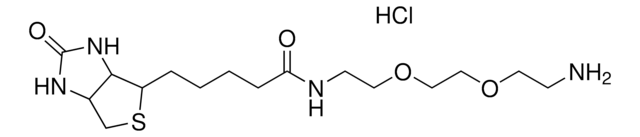
5-(Biotinamido)pentylamine TFA Salt
≥95%
Synonym(s):
N-(5-Aminopentyl)-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pent, Biotin cadaverine TFA, Biotin-DAPe TFA
About This Item
Recommended Products
Quality Level
Assay
≥95%
form
powder
mp
116-121 °C
storage temp.
2-8°C
SMILES string
FC(F)(F)C(=O)O.S1[C@H]([C@H]2NC(=O)N[C@H]2C1)CCCCC(=O)NCCCCCN
InChI key
QWKKJNXOPDNIEU-RGESYUBESA-N
Application
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Other Notes
Convergent synthesis of trifunctional molecules by three sequential azido-type-selective cycloadditions
Locked by Design: A Conformationally Constrained Transglutaminase Tag Enables Efficient Site-Specific Conjugation
Synthesis of Novel Phosphonic-Type Activity-Based Probes for Neutrophil Serine Proteases and Their Application in Spleen Lysates of Different Organisms
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service