689963
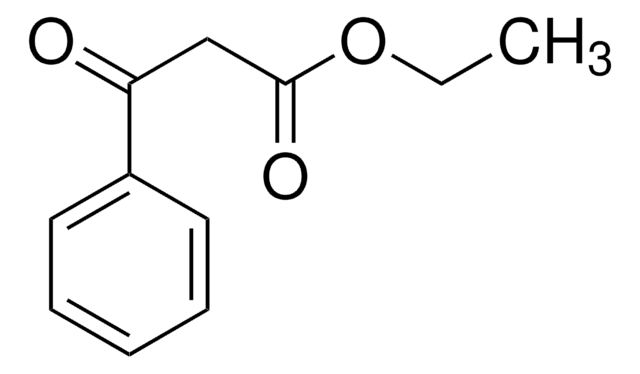
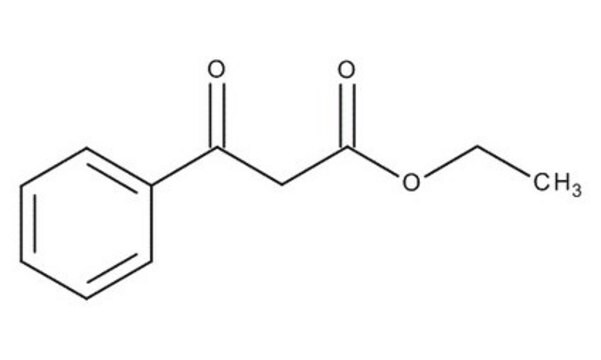
Ethyl benzoylacetate
Arxada quality, ≥98% (GC)
Synonym(s):
Benzoylacetic acid ethyl ester, Ethyl 3-oxo-3-phenylpropionate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5COCH2COOC2H5
CAS Number:
Molecular Weight:
192.21
Beilstein:
389944
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
6.6 (vs air)
Assay
≥98% (GC)
form
liquid
quality
Arxada quality
manufacturer/tradename
Arxada AG
refractive index
n20/D 1.52 (lit.)
bp
265-270 °C (lit.)
density
1.11 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)CC(=O)c1ccccc1
InChI
1S/C11H12O3/c1-2-14-11(13)8-10(12)9-6-4-3-5-7-9/h3-7H,2,8H2,1H3
InChI key
GKKZMYDNDDMXSE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Ethyl benzoylacetate is a general reagent used in the synthesis of substituted pyrroles, oxazoles, indoles, imidazo[1, 2-a] pyridines and a variety of other aromatic compounds.
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
284.0 °F - closed cup
Flash Point(C)
140 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Facile access to polysubstituted indoles via a cascade Cu-catalyzed arylation? condensation process.
Chen Y, et al.
The Journal of Organic Chemistry, 72(24), 9329-9334 (2007)
Facile synthesis of polysubstituted oxazoles via a copper-catalyzed tandem oxidative cyclization.
Wan C, et al.
Organic Letters, 12(10), 2338-2341 (2010)
Rhenium-and manganese-catalyzed synthesis of aromatic compounds from 1, 3-dicarbonyl compounds and alkynes.
Kuninobu Y, et al.
The Journal of Organic Chemistry, 75(2), 334-341 (2009)
TBAI-catalyzed oxidative coupling of aminopyridines with β-keto esters and 1, 3-diones?synthesis of imidazo [1, 2-a] pyridines.
Ma L, et al.
Chemical Communications (Cambridge, England), 47(40), 11333-11335 (2011)
Expedient synthesis of highly substituted pyrroles via tandem rearrangement of α-diazo oxime ethers.
Jiang Y, et al.
Journal of the American Chemical Society, 134(9), 4104-4107 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service