537349
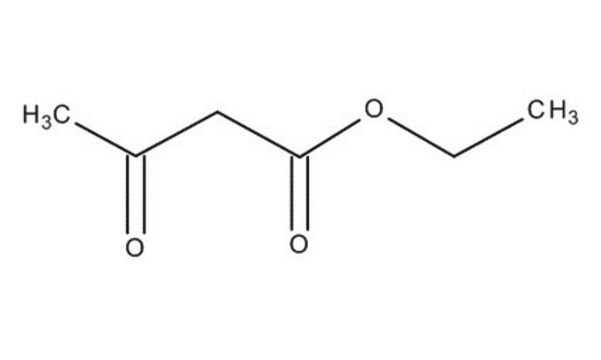
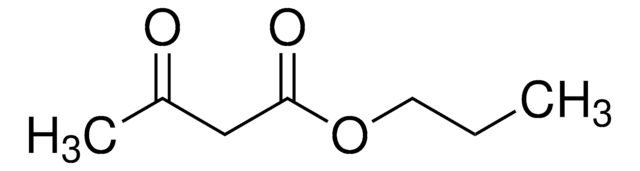
Ethyl acetoacetate
ReagentPlus®, 99%
Synonym(s):
EAA, Ethyl 3-oxobutanoate, Acetoacetic ester
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3COCH2COOC2H5
CAS Number:
Molecular Weight:
130.14
Beilstein:
385838
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
4.48 (vs air)
Quality Level
vapor pressure
1 mmHg ( 28.5 °C)
product line
ReagentPlus®
Assay
99%
autoignition temp.
580 °F
expl. lim.
9.5 %
bp
181 °C (lit.)
mp
−43 °C (lit.)
solubility
water: soluble 130 g/L at 20 °C
density
1.029 g/mL at 20 °C (lit.)
SMILES string
CCOC(=O)CC(C)=O
InChI
1S/C6H10O3/c1-3-9-6(8)4-5(2)7/h3-4H2,1-2H3
InChI key
XYIBRDXRRQCHLP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Ethyl acetoacetate is a versatile reagent that can be used as a nucleophile in alkylation, conjugate addition and condensation reactions. Some of its applications are:
- Alkylation at the α-carbon of ethyl acetoacetate followed by hydrolysis and decarboxylation can afford a variety of methyl ketones.
- It also undergoes acylation at the α-carbon in the presence of MgCl2 and pyridine to give synthetically important intermediates.
- It can be used in Knoevenagel condensation with aliphatic, aromatic, and heteroaromatic aldehydes to produce α-alkylideneacetoacetates.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
164.3 °F - closed cup
Flash Point(C)
73.5 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ethyl Acetoacetate.
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Synthetic Applications of Dealkoxycarbonylations of Malonate Esters, β-Keto Esters, α-Cyano Esters and Related Compounds in Dipolar Aprotic Media-Part II.
Krapcho A P
Synthesis, 1982(11), 893-914 (1982)
Knoevenagel-kondensationen mit titantetrachlorid/base?II: Alkyliden-und arylidenacet-bzw.-nitroessigester bei 0?22?.
Lehnert W
Tetrahedron, 28(3), 663-666 (1972)
Procedures for the acylation of diethyl malonate and ethyl acetoacetate with acid chlorides using tertiary amine bases and magnesium chloride.
Rathke MW and Cowan PJ.
The Journal of Organic Chemistry, 50(15), 2622-2624 (1985)
Gui-Rong Qu et al.
Organic letters, 11(8), 1745-1748 (2009-03-20)
A novel approach to the synthesis of purines bearing functionalized carbon substituents or methyl in position 6 was developed. Under different reaction conditions, 6-halopurine derivatives could react with ethyl acetoacetate efficiently to yield 2-(purin-6-yl)acetoacetic acid ethyl esters, (purin-6-yl)acetates and 6-methylpurines
Articles
Biginelli Reaction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service