1604803
USP
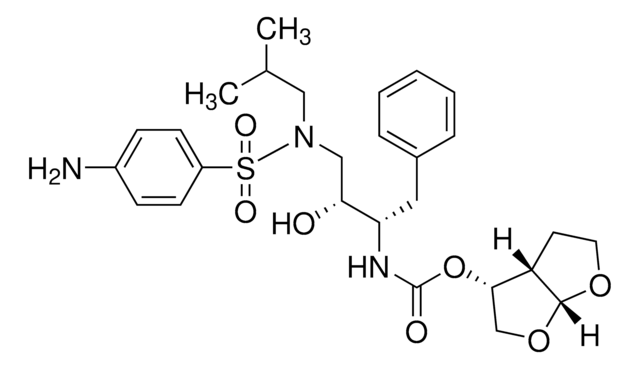
Ritonavir
United States Pharmacopeia (USP) Reference Standard
Synonym(e):
1,3-Thiazol-5-ylmethyl-N-[(2S,3S,5S)-3-hydroxy-5-[[(2S)-3-methyl-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)-methyl]-carbamoyl]-amino]-butanoyl]-amino]-1,6-diphenylhexan-2-yl]-carbamat
About This Item
Empfohlene Produkte
Biologische Quelle
synthetic
Qualität
pharmaceutical primary standard
Agentur
USP
Dampfdruck
<0.0000001 kPa ( 25 °C)
API-Familie
ritonavir
Form
powder
Verpackung
pkg of 200 mg
Hersteller/Markenname
USP
Lagerbedingungen
protect from light
Farbe
white to tan
Löslichkeit
acetonitrile: slightly soluble
ethanol: freely soluble
methanol: freely soluble
methylene chloride: freely soluble
water: practically insoluble
Anwendung(en)
pharmaceutical (small molecule)
Format
neat
InChI
1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1
InChIKey
NCDNCNXCDXHOMX-XGKFQTDJSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Ritonavir belongs to the group of protease inhibitors that are widely used in combination with other drugs in the prevention of HIV. Its mode of action involves binding to the active site of the protease enzyme and preventing the further maturation of new viral particles.
Anwendung
Also used to prepare standard, standard stock, identification, and standard working solution for assay, impurity analysis, and performance test according to the given below monographs of United States Pharmacopeia (USP):
- Lopinavir and Ritonavir Tablets
- Ritonavir Tablets
- Ritonavir
- Lopinavir and Ritonavir Oral Solution
Hinweis zur Analyse
Sonstige Hinweise
Ähnliches Produkt
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.