Wichtige Dokumente
1445470
USP
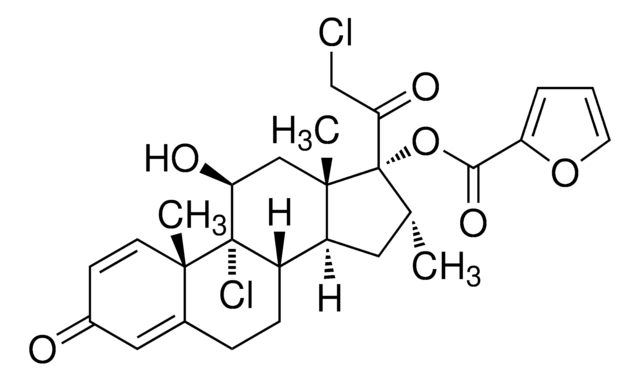
Mometasonfuroat
United States Pharmacopeia (USP) Reference Standard
Synonym(e):
(11β,16α)-9,21-Dichlor-17-[(2-furanylcarbonyl)-oxy]-11-hydroxy-16-methylpregna-1,4-dien-3,20-dion
About This Item
Empfohlene Produkte
Qualität
pharmaceutical primary standard
API-Familie
mometasone
Hersteller/Markenname
USP
Anwendung(en)
pharmaceutical (small molecule)
Format
neat
SMILES String
C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@]4(C)[C@@]3(Cl)[C@@H](O)C[C@]2(C)[C@@]1(OC(=O)c5ccco5)C(=O)CCl
InChI
1S/C27H30Cl2O6/c1-15-11-19-18-7-6-16-12-17(30)8-9-24(16,2)26(18,29)21(31)13-25(19,3)27(15,22(32)14-28)35-23(33)20-5-4-10-34-20/h4-5,8-10,12,15,18-19,21,31H,6-7,11,13-14H2,1-3H3/t15-,18+,19+,21+,24+,25+,26+,27+/m1/s1
InChIKey
WOFMFGQZHJDGCX-ZULDAHANSA-N
Angaben zum Gen
human ... NR3C1(2908)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Hinweis zur Analyse
Sonstige Hinweise
Ähnliches Produkt
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Repr. 1B
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.