Wichtige Dokumente
1066009
USP
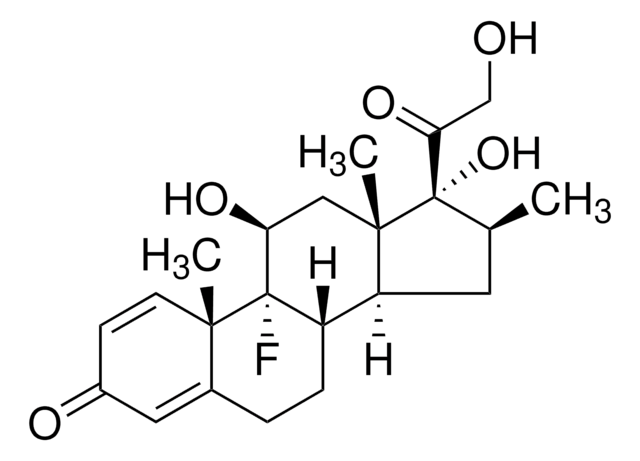
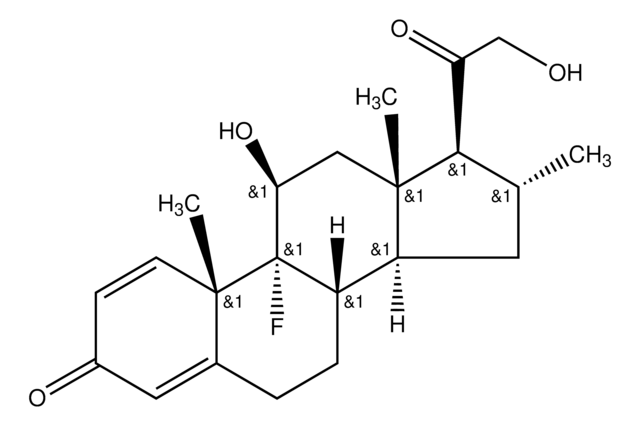
Betamethason
United States Pharmacopeia (USP) Reference Standard
Synonym(e):
9α-Fluor-11β,17α,21-trihydroxy-16β-methylpregna-1,4-dien-3,20-dion, 9α-Fluor-16β-methyl-11β,17α,21-trihydroxy-1,4-pregnadien-3,20-dion, 9α-Fluor-16β-methylprednisolon
About This Item
Empfohlene Produkte
Biologische Quelle
synthetic
Qualität
pharmaceutical primary standard
Agentur
USP
Dampfdruck
<0.0000001 kPa ( 25 °C)
API-Familie
betamethasone
Verpackung
pkg of 200 mg
Hersteller/Markenname
USP
Farbe
white to off-white
mp (Schmelzpunkt)
235-237 °C (lit.)
447.8-473 °F (231—245°C; decomposes)
Löslichkeit
acetone: sparingly soluble
chloroform: very slightly soluble
ethanol: sparingly soluble
ether: very slightly soluble
methanol: sparingly soluble
water: insoluble
Dichte
0.305 g/cm3 at 25 °C (77°F)
Anwendung(en)
pharmaceutical (small molecule)
Format
neat
Lagertemp.
2-8°C
SMILES String
[H][C@@]12C[C@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]3(F)[C@@]2([H])CCC4=CC(=O)C=C[C@]34C
InChI
1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15-,16-,17-,19-,20-,21-,22-/m0/s1
InChIKey
UREBDLICKHMUKA-DVTGEIKXSA-N
Angaben zum Gen
human ... NR3C1(2908)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Anwendung
- Betamethasone Acetate
- Betamethasone Oral Solution
- Betamethasone Valerate Cream
- Betamethasone Valerate Lotion
- Betamethasone Valerate Ointment
- Dexamethasone
Hinweis zur Analyse
Sonstige Hinweise
Ähnliches Produkt
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Repr. 1B - STOT RE 2
Zielorgane
Liver,Kidney,Endocrine system
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Protokolle
A simple, precise and sensitive Reverse-Phase High Pressure Liquid Chromatography gradient method was adapted for traceability, homogeneity and total chromatographic analysis of Dexamethasone. The given experimental conditions follow the USP43-NF38 monograph method for Dexamethasone Assay and Organic Impurity Profiling. Dexamethasone, Betamethasone, Dexamethasone acetate and Desoximetasone were baseline resolved within 20 minutes using a Titan C18 UHPLC column (2.1 x 100 mm, 1.9 µm).
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.