G005819
LC/MS (TOF) Analysis of Antiarrhythmic Drugs and Metabolites on Ascentis® Express HILIC: Improvement in Analyte Response after Solid Phase Extraction (SPE) using HybridSPE®-Phospholipid
application for HPLC, application for SPE
About This Item
Empfohlene Produkte
Methode(n)
HPLC: suitable
solid phase extraction (SPE): suitable
Testparameter
sample preparation: SPE (Solid Phaes Extraction)
sample: rat plasma was spiled directly from stock standard to a level of 400 ng/mL
SPE tube/cartridge: HybridSPE-Phospholipid 96-Well Plate, 50 mg/2 mL (575656-U)
sample addition: apply 100 μL of plasma to plate, followed by 300 μL of 1% formic acid acetonitrile. Agitate via vortex for 4 min
elution: place on vacuum manifold and apply 10" Hg vacuum for 4 minutes. Collect filtrate and analyze directly.
column: Ascentis Express HILIC, 10 cm x 2.1 mm I.D., 2.7 μm particles (53939-U)
mobile phase: [A] 5 mM ammonium formate; [B] 5 mM ammonium formate in acetonitrile; (5:95, A:B, pH 7.0 with formic acid)
flow rate: 0.4 mL/min
pressure: 1305 psi (90 bar)
column temp.: 35 °C
detector: ESI(+), full scan, m/z 100-1000
injection: 0.5 μL
sample: plasma extract, analyte concentration of final sample work-up is equivalent to 100 ng/mL
Eignung
application for HPLC
application for SPE
Anwendung(en)
clinical
Hinweis zur Analyse
Rechtliche Hinweise
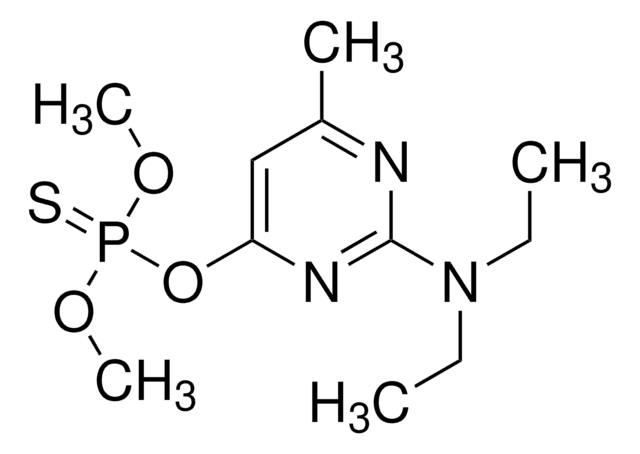
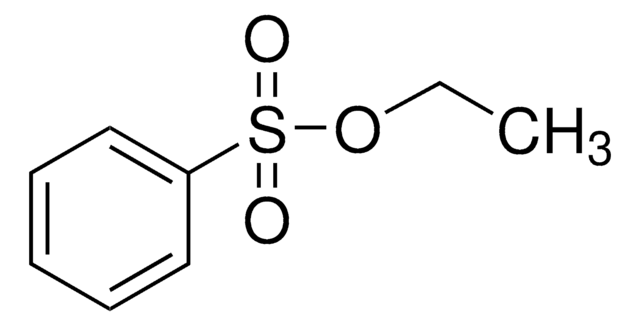
Analyt
Analytische Säule
SPE-Röhrchen oder -Platte
Standard
Zubehör
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.