Wichtige Dokumente
SMB01016
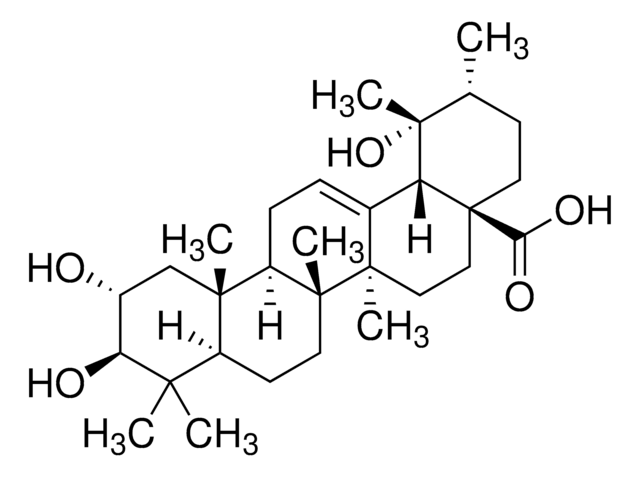
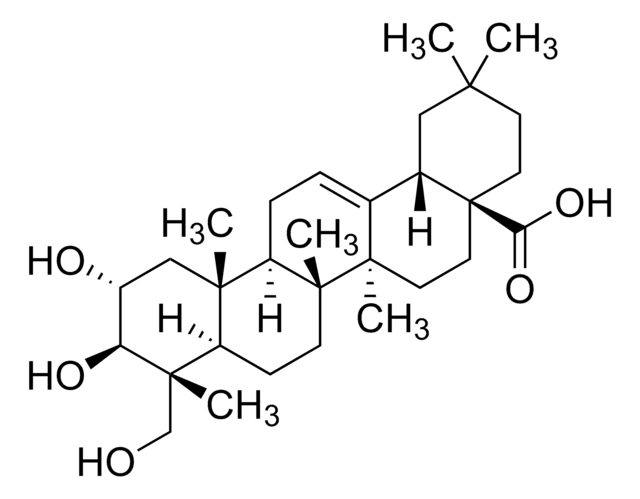
Pomolic acid
≥90% (LC/MS-ELSD)
Synonym(e):
19α-Hydroxyursolic acid, Benthamic acid, Randialic acid A
About This Item
Empfohlene Produkte
Biologische Quelle
plant
Assay
≥90% (LC/MS-ELSD)
Form
solid
Mol-Gew.
472.7
Löslichkeit
water: slightly soluble
Anwendung(en)
metabolomics
vitamins, nutraceuticals, and natural products
Lagertemp.
−20°C
SMILES String
O[C@]1([C@@H]2[C@@](CC[C@]3([C@]4([C@@H]([C@@]5([C@H](C([C@H](CC5)O)(C)C)CC4)C)CC=C32)C)C)(CC[C@H]1C)C(=O)O)C
InChI
1S/C30H48O4/c1-18-10-15-30(24(32)33)17-16-27(5)19(23(30)29(18,7)34)8-9-21-26(4)13-12-22(31)25(2,3)20(26)11-14-28(21,27)6/h8,18,20-23,31,34H,9-17H2,1-7H3,(H,32,33)/t18-,20+,21-,22+,23-,26+,27-,28-,29-,30+/m1/s1
InChIKey
ZZTYPLSBNNGEIS-OPAXANQDSA-N
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Leistungsmerkmale und Vorteile
- Suitable for Biochemical and Biomedical research
- Versatile and adaptable for wide variety of laboratory and research applications
Sonstige Hinweise
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.