Wichtige Dokumente
M3935
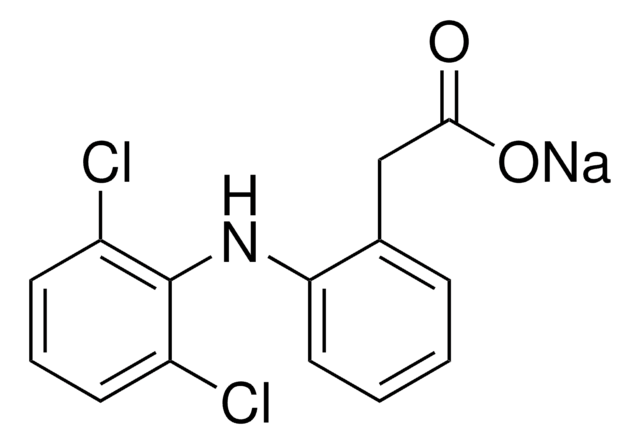
Meloxicam sodium salt hydrate
≥98% (HPLC)
Synonym(e):
4-Hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide sodium hydrate, Metacam sodium salt hydrate, Mobec sodium salt hydrate, UH-AC 62XX sodium salt hydrate
About This Item
Empfohlene Produkte
Assay
≥98% (HPLC)
Form
powder
Farbe
light yellow to dark yellow
Löslichkeit
DMSO: 5 mg/mL, clear
Ersteller
Boehringer Ingelheim
Lagertemp.
room temp
SMILES String
O.[Na+].CN1C(C(=O)Nc2ncc(C)s2)=C([O-])c3ccccc3S1(=O)=O
InChI
1S/C14H13N3O4S2.Na.H2O/c1-8-7-15-14(22-8)16-13(19)11-12(18)9-5-3-4-6-10(9)23(20,21)17(11)2;;/h3-7,18H,1-2H3,(H,15,16,19);;1H2/q;+1;/p-1
InChIKey
IZBZAOZGNNQKPJ-UHFFFAOYSA-M
Angaben zum Gen
human ... PTGS2(5743)
Anwendung
- as a cyclooxygenase-2 (COX-2) inhibitor in solid Ehrlich tumor
- as an alternative to diclofenac nonsteroidal anti-inflammatory drugs (NSAID) to treat vultures
- as a reference standard in liquid chromatography electrospray ionisation mass spectrometry (LC-ESI/MS)
- to test its effect on prostaglandin (PGE2) production in lipopolysaccharide (LPS)-induced COX-2 protein expression in RAW246.7 cells
Biochem./physiol. Wirkung
Leistungsmerkmale und Vorteile
Ähnliches Produkt
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.