HPA022132
Anti-CD38 antibody produced in rabbit

Prestige Antibodies® Powered by Atlas Antibodies, affinity isolated antibody, buffered aqueous glycerol solution
Synonym(e):
Anti-ADP-ribosyl cyclase 1, Anti-CD38 antigen, Anti-Cyclic ADP-ribose hydrolase 1, Anti-T10, Anti-cADPr hydrolase 1
About This Item
Empfohlene Produkte
Biologische Quelle
rabbit
Qualitätsniveau
Konjugat
unconjugated
Antikörperform
affinity isolated antibody
Antikörper-Produkttyp
primary antibodies
Klon
polyclonal
Produktlinie
Prestige Antibodies® Powered by Atlas Antibodies
Form
buffered aqueous glycerol solution
Speziesreaktivität
human
Erweiterte Validierung
orthogonal RNAseq
recombinant expression
Learn more about Antibody Enhanced Validation
Methode(n)
immunoblotting: 0.04-0.4 μg/mL
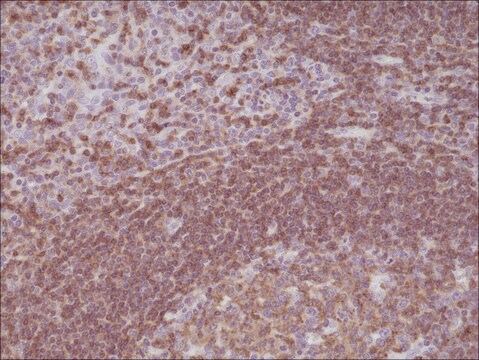
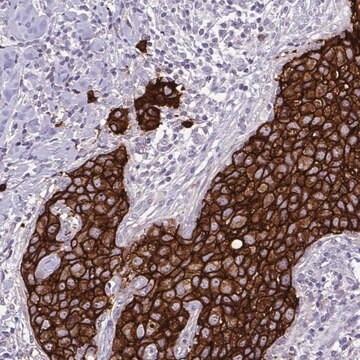
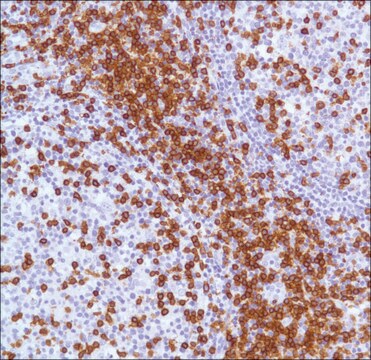
immunohistochemistry: 1:1000-1:2500
Immunogene Sequenz
AACDVVHVMLNGSRSKIFDKNSTFGSVEVHNLQPEKVQTLEAWVIHGGREDSRDLCQDPTIKELESIISKRNIQFSCKNIYRPDKFLQCVKNPEDSSCTSEI
UniProt-Hinterlegungsnummer
Anwendung(en)
research pathology
Versandbedingung
wet ice
Lagertemp.
−20°C
Posttranslationale Modifikation Target
unmodified
Angaben zum Gen
human ... CD38(952)
Immunogen
Anwendung
The Human Protein Atlas project can be subdivided into three efforts: Human Tissue Atlas, Cancer Atlas, and Human Cell Atlas. The antibodies that have been generated in support of the Tissue and Cancer Atlas projects have been tested by immunohistochemistry against hundreds of normal and disease tissues and through the recent efforts of the Human Cell Atlas project, many have been characterized by immunofluorescence to map the human proteome not only at the tissue level but now at the subcellular level. These images and the collection of this vast data set can be viewed on the Human Protein Atlas (HPA) site by clicking on the Image Gallery link. We also provide Prestige Antibodies® protocols and other useful information.
Leistungsmerkmale und Vorteile
Every Prestige Antibody is tested in the following ways:
- IHC tissue array of 44 normal human tissues and 20 of the most common cancer type tissues.
- Protein array of 364 human recombinant protein fragments.
Verlinkung
Physikalische Form
Rechtliche Hinweise
Haftungsausschluss
Sie haben nicht das passende Produkt gefunden?
Probieren Sie unser Produkt-Auswahlhilfe. aus.
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.