Alle Fotos(1)
Wichtige Dokumente
56224
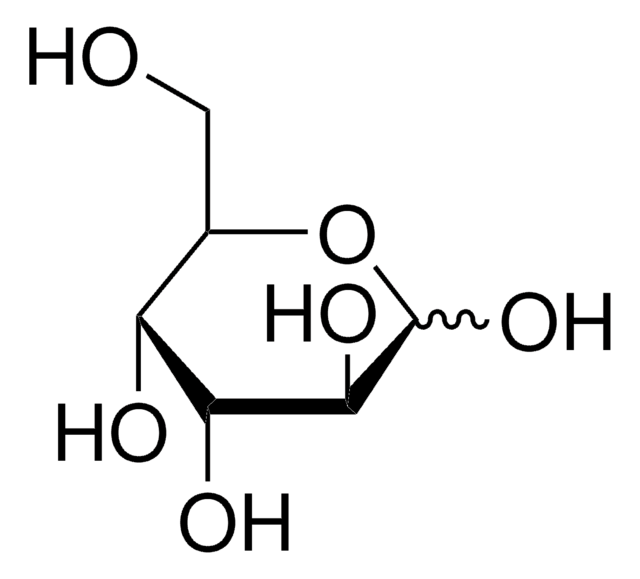
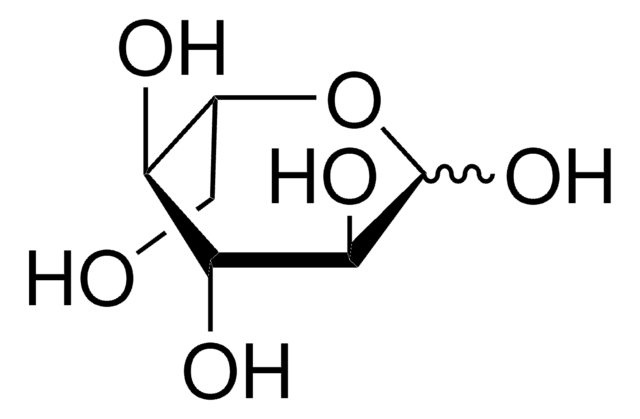
L-(+)-Gulose
≥98.0% (HPLC)
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C6H12O6
CAS-Nummer:
Molekulargewicht:
180.16
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352201
PubChem Substanz-ID:
NACRES:
NA.25
Empfohlene Produkte
Assay
≥98.0% (HPLC)
Optische Aktivität
[α]/D +23.0±2°, 24 hr, c = 0.5% in H2O
Lagertemp.
room temp
SMILES String
OC[C@@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3+,4-,5-,6?/m0/s1
InChIKey
WQZGKKKJIJFFOK-QRXFDPRISA-N
Anwendung
L-(+)-Gulose, a rare aldohexose sugar C-3 epimer of galactose, is used in the development of various drugs and to glycosylate alginates. L-(+)-Gulose is a substrate of GDP-mannose 3′,5′-epimerase in plants and may be a precursor of L-ascorbic acid in plants.
Sonstige Hinweise
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Daniele Castagnolo et al.
Carbohydrate research, 344(11), 1285-1288 (2009-06-09)
An efficient and stereoselective synthesis of D,L-gulose was described. The key step of the synthetic route is represented by a multicomponent enyne cross metathesis-hetero Diels-Alder reaction which allows the formation of the pyran ring from cheap and commercially available substrates
Jasper Dinkelaar et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(30), 9400-9411 (2008-09-05)
The glycosylation properties of gulopyranosides have been mapped out, and it is shown that gulose has an intrinsic preference for the formation of 1,2-cis-glycosidic bonds. It is postulated that this glycosylation behaviour originates from nucleophilic attack at the oxacarbenium ion
Yunshan Sun et al.
The Journal of organic chemistry, 77(17), 7401-7410 (2012-08-03)
A versatile synthesis of orthogonally protected derivatives of carba-α-D-glucosamine, carba-α-D-mannose, carba-α-D-mannuronic acid, carba-β-L-idosamine, and carba-β-L-gulose from methyl α-D-mannoside is described. Our synthetic strategy utilizes the palladium-promoted Ferrier carbocyclization and persistent butane-2,3-diacetal protection to produce a key chiral cyclohexanone intermediate, from
Saikat Dutta et al.
ACS applied materials & interfaces, 4(3), 1560-1564 (2012-02-18)
Self-assembled TiO(2) nanoparticulate materials with well-defined spherical morphologies were synthesized by using a biopolymer sodium alginate as a template under different synthesis conditions. Powder X-ray diffraction (XRD), transmission electron microscopy (TEM), and scanning electron microscopy (SEM) techniques were used to
Y Zhu et al.
The Journal of organic chemistry, 66(19), 6244-6251 (2001-09-18)
High-resolution (13)C NMR spectra (150 MHz) have been obtained on the complete series of D-aldohexoses (D-allose 1, D-altrose 2, D-galactose 3, D-glucose 4, D-gulose 5, D-idose 6, D-mannose 7, D-talose 8) selectively labeled with (13)C at C1 in order to
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.