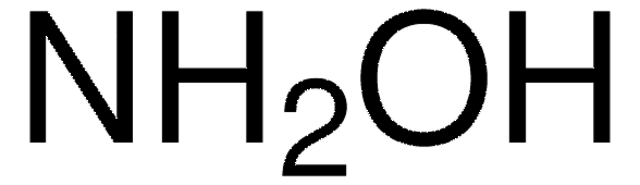159417
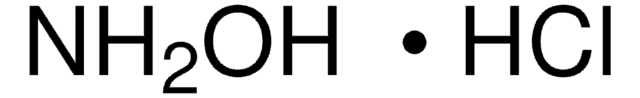
Hydroxylamin -hydrochlorid
ReagentPlus®, 99%
Synonym(e):
Hydroxylammoniumchlorid
About This Item
Empfohlene Produkte
Dampfdruck
0.001 hPa ( 50 °C)
Qualitätsniveau
Produktlinie
ReagentPlus®
Assay
99%
Form
crystalline
Methode(n)
inhibition assay: suitable
pH-Wert
2.5-3.5 (20 °C, 50 g/L)
mp (Schmelzpunkt)
155-157 °C (dec.) (lit.)
Dichte
1.67 g/mL at 25 °C (lit.)
SMILES String
Cl.NO
InChI
1S/ClH.H3NO/c;1-2/h1H;2H,1H2
InChIKey
WTDHULULXKLSOZ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- in the synthesis of primary amides from aldehydes in the presence of cesium carbonate (Cs2CO3) as a catalyst.
- in the conversion of alicyclic /aliphatic carbonyl compounds and the aromatic aldehydes into corresponding oximes.
- in the one-pot synthesis of nitriles from aldehydes in the presence of sodium sulfate (anhyd) and sodium bicarbonate catalysts.
- It can also be used as a reducing agent in the preparation of single-layer reduced graphene oxide (RGO) sheets and films.
Biochem./physiol. Wirkung
Rechtliche Hinweise
Signalwort
Warning
Gefahreneinstufungen
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
Zielorgane
spleen
Lagerklassenschlüssel
4.1A - Other explosive hazardous materials
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.