Wichtige Dokumente
43720
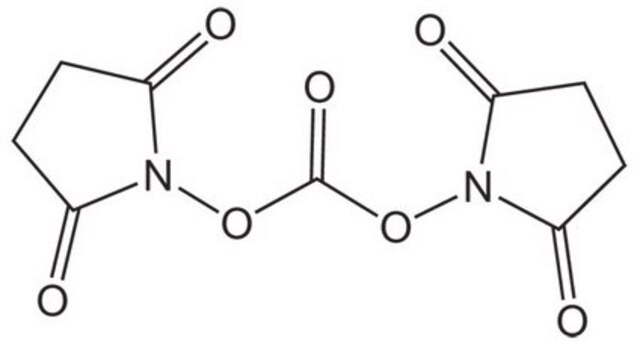
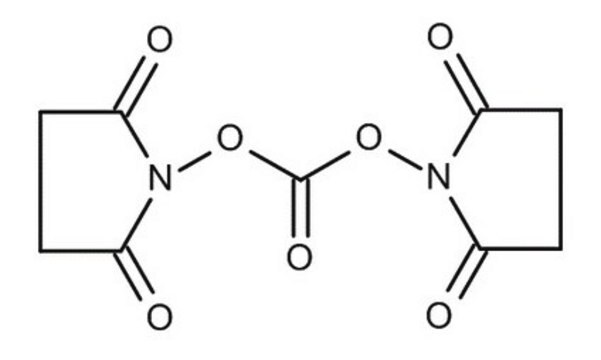
Di-(N-succinimidyl)-carbonat
≥95.0% (NMR), for peptide synthesis
Synonym(e):
N-Succinimidyl-carbonat, DSC
About This Item
Empfohlene Produkte
Produktbezeichnung
Di-(N-succinimidyl)-carbonat, purum, ≥95.0% (NMR)
Qualität
purum
Qualitätsniveau
Assay
≥95.0% (NMR)
Form
powder
Eignung der Reaktion
reaction type: Carbonylations
Verunreinigungen
~3% N-hydroxysuccinimide (NMR)
mp (Schmelzpunkt)
190 °C (dec.) (lit.)
Anwendung(en)
peptide synthesis
Funktionelle Gruppe
imide
Lagertemp.
−20°C
SMILES String
O=C1CCC(=O)N1OC(=O)ON2C(=O)CCC2=O
InChI
1S/C9H8N2O7/c12-5-1-2-6(13)10(5)17-9(16)18-11-7(14)3-4-8(11)15/h1-4H2
InChIKey
PFYXSUNOLOJMDX-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
- Various carbamate derivatives from primary and sterically hindered secondary alcohols by alkoxycarbonylation.
- Active carbonate resins from 4-hydroxymethylpolystyrene and 4-hydroxymethyl-3-nitrobenzamido resins via hydroxy functional groups.
- Aza-glycinyl dipeptides, important intermediates for the preparation of various azapeptides.
It may be also used:
- In the two-step preparation of 5-(6-(azidomethyl)nicotinamido)pentanoic acid, a copper-chelating picolyl azide derivative.
- To activate the hydroxyl group of the hapten, γ-hydroxyphenylbutazone (HPBZ) so that HPBZ can effectively bind with human serum albumin(HSA)-immunogen to form a hapten-protein conjugate.
Sonstige Hinweise
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT RE 2 Oral
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
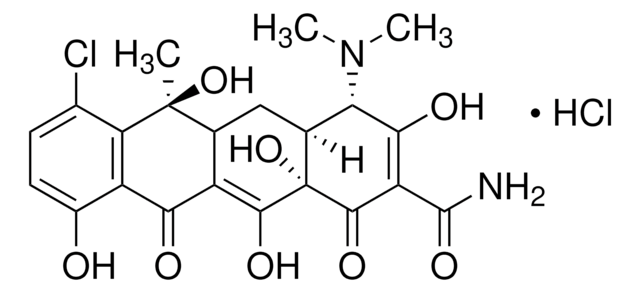
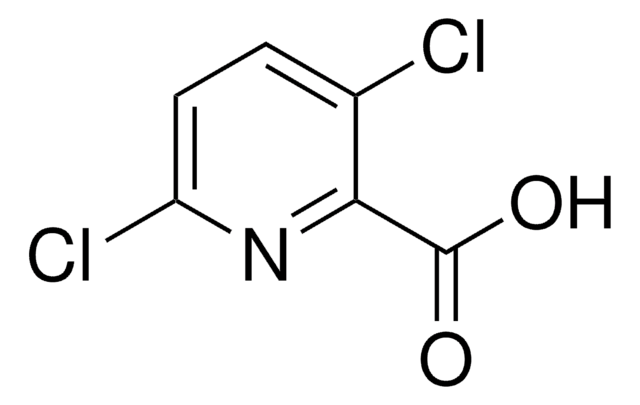
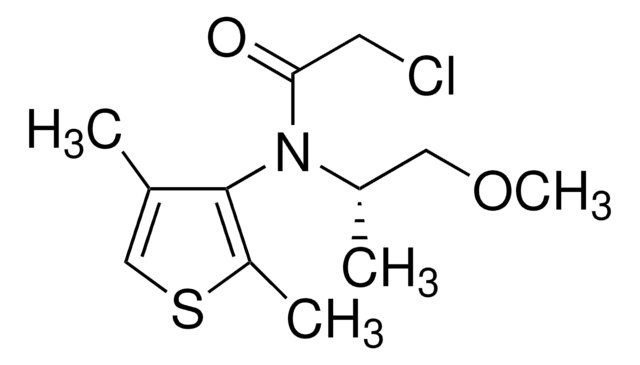
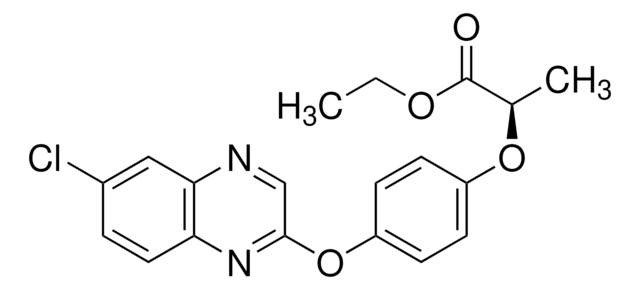
Kunden haben sich ebenfalls angesehen
Artikel
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
In principle, the seemingly simple formation of a peptide bond can be accomplished using all the procedures available in organic chemistry for the synthesis of carboxylic acid amides. However, due to the presence of various functional groups in natural and unnatural amino acids and particularly the requirement for full retention of chiral integrity, the coupling of amino acids and peptides under mild conditions can be challenging. A plethora of coupling reagents has been developed superseding each other in efficiency and suitability for specific applications (e.g., solid-phase peptide synthesis or fragment condensation).
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.