TZHVDV205
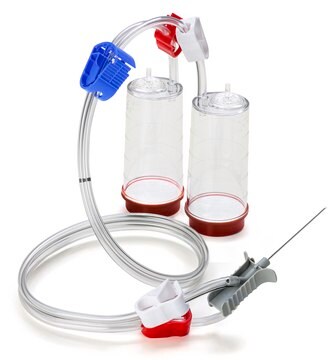
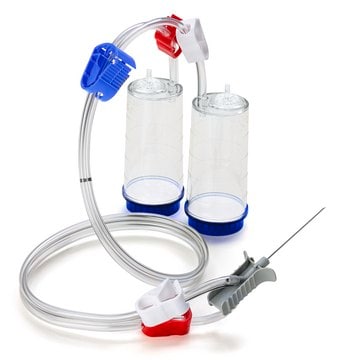
Steritest® NEO Device
For soluble powders in vials with septa., Red base canister with vented double needle for small vials with septa., pkg of 10 blisters in 2 bags of 5 blisters per box, Double packed
Synonym(e):
Red Base Steritest® NEO device for sterility testing, Sterility testing device, membrane filtration device, membrane filtration canister, closed membrane filtration
About This Item
Empfohlene Produkte
Materialien
Nylon 66 adapter (for needle)
PVC tubing (double lumen)
PVDF membrane
plain filter
stainless steel (for needle)
styrene-acrylonitrile (SAN) (for canister)
Qualitätsniveau
Agentur
EP (2.6.1)
JP (4.06)
USP 71
Sterilität
sterile; γ-irradiated
Leistungsmerkmale
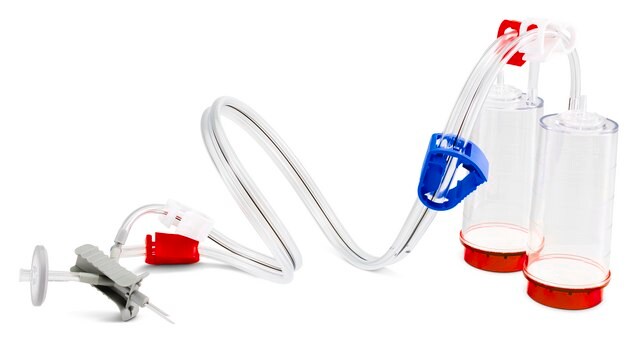
Red base canister with vented double needle for small vials with septa.
Hersteller/Markenname
Steritest®
Verpackung
pkg of 10 blisters in 2 bags of 5 blisters per box, Double packed
Parameter
120 mL sample volume (graduation marks at 25, 50, 75 and 100 mL)
3.1 bar max. inlet pressure (45 psi) at 25 °C
45 °C max. temp.
Länge Schlauch
850 mm
Farbe
red Canister Base
Matrix
Durapore®
Porengröße
0.45 μm pore size
Aufnahme
sample type pharmaceutical(s)
Anwendung(en)
pharmaceutical
sterility testing
Kompatibilität
For soluble powders in vials with septa.
Versandbedingung
ambient
Verwandte Kategorien
Allgemeine Beschreibung
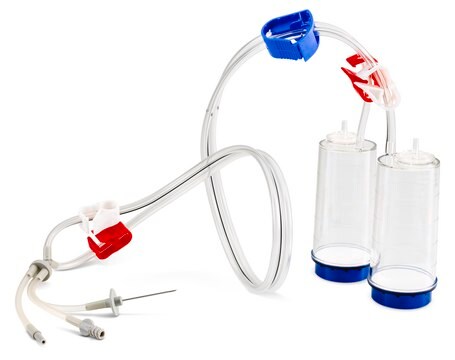
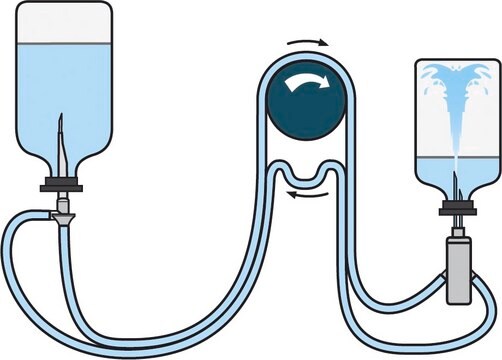
Steritest® NEO is a membrane filtration device for sterility testing of filterable pharmaceutical products. The device simplifies every aspect of testing, from handling to traceability. The closed system ensures that pharmaceutical products are never exposed to the environment, minimizes false positives, and offers the highest levels of quality & reliability. This test system offers an optimized and fully regulatory compliant testing process when used with the Steritest® Symbio pump, specific accessories and high-quality culture media and rinsing fluids. The device is gamma sterilized and double packed for the quick transfer into sterility testing environments, simplifying decontamination procedures and saving time. The device simultaneously dissolves/dilutes the sample in sterile diluent and transfers the resulting solution to canisters. A small diameter double needle adapter is used for small vials with septum. The red canister base indicates low absorption. Durapore® hydrophilic Poly vinylidene fluoride (PVDF) membrane and specific drain design. This optimizes the rinsing of products that inhibit microbial growth.
Anwendung
Leistungsmerkmale und Vorteile
- One-stop-shop for sterility testing with our devices, pumps, media, fluids, and services
- Steritest® devices are manufactured in our Center of Excellence in Molsheim, France, with high-quality control standards maintaining the Certificate of Quality for each lot.
- New needle design
- Smarter workflow
- Completely closed set up
- Consistent performance
- New tubing disconnection tool
Verpackung
Rechtliche Hinweise
konfiguriert für
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.