Alle Fotos(1)
Wichtige Dokumente
I17451
Isonicotinsäureamid
ReagentPlus®, 99%
Synonym(e):
Isonicotinamid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C6H6N2O
CAS-Nummer:
Molekulargewicht:
122.12
Beilstein:
2173
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Produktlinie
ReagentPlus®
Assay
99%
mp (Schmelzpunkt)
155-157 °C (lit.)
SMILES String
NC(=O)c1ccncc1
InChI
1S/C6H6N2O/c7-6(9)5-1-3-8-4-2-5/h1-4H,(H2,7,9)
InChIKey
VFQXVTODMYMSMJ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
Isonicotinamide (pyridine-4-carboxamide) can be used as a heterocyclic building block to synthesize:
It can also be used as a co-former with active pharmaceutical ingredients (APIs) to prepare co-crystals.
- 4-oxo-1,3-thiazinan-3-yl isonicotinamide derivatives as potential anti-tubercular agents.
- Organotin(IV) complexes of isonicotinamide via synthesis of phosphoramidate ligands for various biological activity studies.
- Bis-pyridinium isonicotinamide derivatives of 2-(hydroxyimino)-N-(pyridin-3-yl)acetamide as potent reactivators sarin.
It can also be used as a co-former with active pharmaceutical ingredients (APIs) to prepare co-crystals.
Rechtliche Hinweise
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Gareth Arnott et al.
Organic letters, 10(14), 3089-3092 (2008-06-17)
Treatment of N-arylisonicotinamides with trifluoromethanesulfonic anhydride triggers intramolecular nucleophilic attack of the aryl ring on the 4-position of the pyridinium intermediate. The products are spirocyclic dihydropyridines which can be converted to valuable spirocyclic piperidines related to biologically active molecules such
Rodrigo A de Souza et al.
European journal of medicinal chemistry, 45(11), 4863-4868 (2010-08-21)
Complexes of the type trans-[PdX(2)(isn)(2)] {X = Cl (1), N(3) (2), SCN (3), NCO (4); isn = isonicotinamide} were synthesized and evaluated for in vitro antimycobacterial and antitumor activities. The coordination mode of the isonicotinamide and the pseudohalide ligands was
Jemma Senczyszyn et al.
Organic letters, 15(8), 1922-1925 (2013-04-04)
On treatment with acylating or sulfonylating agents, N-alkenyl pyridine carboxamides (N-pyridinecarbonyl enamines) undergo a dearomatizing cyclization initiated by pyridine acylation and followed by intramolecular trapping of the resulting pyridinium cation. The products are spirocyclic dihydropyridines which may be further elaborated
Sarah Boyd et al.
Journal of pharmaceutical sciences, 99(9), 3779-3786 (2010-07-29)
The solubility and crystal growth of the 1:1 cocrystal between benzoic acid and isonicotinamide from 95% ethanol was studied through the creation of a ternary phase diagram at differing temperatures and turbidity measurements. From the solubility measurements thermodynamic properties of
Shaun R Stauffer et al.
Bioorganic & medicinal chemistry letters, 17(6), 1788-1792 (2007-01-30)
A series of low-molecular weight 2,6-diamino-isonicotinamide BACE-1 inhibitors containing an amine transition-state isostere were synthesized and shown to be highly potent in both enzymatic and cell-based assays. These inhibitors contain a trans-S,S-methyl cyclopropane P(3) which bind BACE-1 in a 10s-loop
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.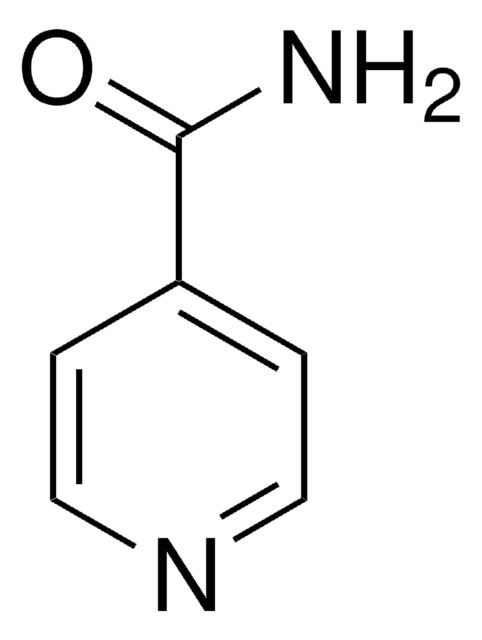





![6-Methyl-1,3-dihydrofuro[3,4-c]pyridin-7-ol -hydrochlorid Pharmaceutical Secondary Standard; Certified Reference Material](/deepweb/assets/sigmaaldrich/product/images/802/873/5e9fd09e-b3ee-4032-b4bb-8a49e380fdc0/640/5e9fd09e-b3ee-4032-b4bb-8a49e380fdc0.jpg)