Wichtige Dokumente
914134
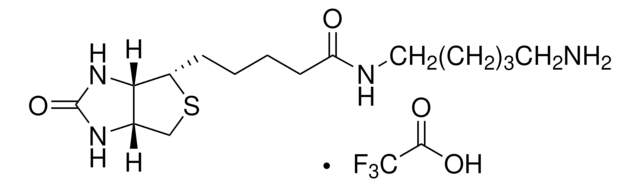
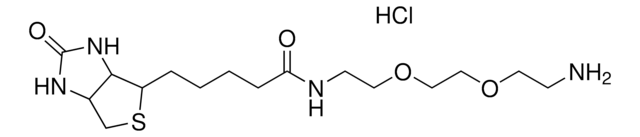
5-(Biotinamido)pentylamine TFA Salt
≥95%
Synonym(e):
N-(5-Aminopentyl)-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pent, Biotin cadaverine TFA, Biotin-DAPe TFA
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥95%
Form
powder
mp (Schmelzpunkt)
116-121 °C
Lagertemp.
2-8°C
SMILES String
FC(F)(F)C(=O)O.S1[C@H]([C@H]2NC(=O)N[C@H]2C1)CCCCC(=O)NCCCCCN
InChIKey
QWKKJNXOPDNIEU-RGESYUBESA-N
Anwendung
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Sonstige Hinweise
Convergent synthesis of trifunctional molecules by three sequential azido-type-selective cycloadditions
Locked by Design: A Conformationally Constrained Transglutaminase Tag Enables Efficient Site-Specific Conjugation
Synthesis of Novel Phosphonic-Type Activity-Based Probes for Neutrophil Serine Proteases and Their Application in Spleen Lysates of Different Organisms
Ähnliches Produkt
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumente section.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.