905313
Rh2(S-TCPTAD)4
Synonym(e):
Davies dirhodium catalyst
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
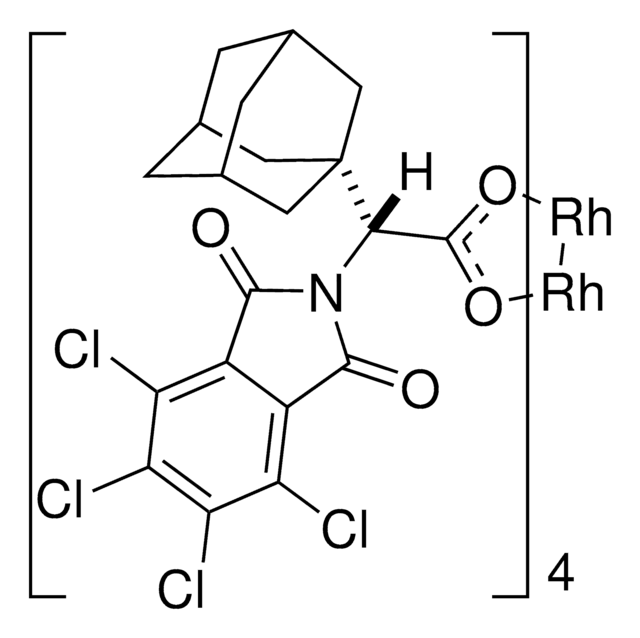
Empirische Formel (Hill-System):
C80H64Cl16N4O16Rh2
CAS-Nummer:
Molekulargewicht:
2110.44
UNSPSC-Code:
12352101
NACRES:
NA.22
Empfohlene Produkte
Form
powder or crystals
Verwandte Kategorien
Anwendung
This dirhodium catalyst developed by the Davies lab can form C-C bonds at the most accessible tertiary C-H position with control of both regioselectivity and absolute configuration.
Sonstige Hinweise
Formation of Tertiary Alcohols from the Rhodium-Catalyzed Reactions of Donor/Acceptor Carbenes with Esters
Harnessing the β-Silicon Effect for Regioselective and Stereoselective Rhodium(II)-Catalyzed C-H Functionalization by Donor/Acceptor Carbenes Derived from 1-Sulfonyl-1,2,3-triazoles
Site-selective and stereoselective functionalization of non-activated tertiary C-H bonds
Harnessing the β-Silicon Effect for Regioselective and Stereoselective Rhodium(II)-Catalyzed C-H Functionalization by Donor/Acceptor Carbenes Derived from 1-Sulfonyl-1,2,3-triazoles
Site-selective and stereoselective functionalization of non-activated tertiary C-H bonds
Ähnliches Produkt
Produkt-Nr.
Beschreibung
Preisangaben
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Lot/Batch Number
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Enantioselective intramolecular aza-spiroannulation onto benzofurans using chiral rhodium catalysis.
Shibuta T, et al.
Heterocycles, 89, 631-639 (2014)
Liangbing Fu et al.
Organic letters, 16(11), 3036-3039 (2014-05-21)
A rhodium-catalyzed asymmetric synthesis of β-lactones via intramolecular C-H insertion into the ester group of aryldiazoacetates has been developed. The β-lactones were synthesized in high yields and with high levels of diastereo- and enantioselectivity. Halo and trifluoromethyl substituents at the
Shigeki Sato et al.
Chemical communications (Cambridge, England), (41), 6264-6266 (2009-10-15)
A versatile, highly enantiocontrolled entry to the spiro-beta-lactam core of chartellines has been developed by expanding the scope of oxidative nitrogen atom transfer methodology based on chiral Rh-nitrenoid species.
Liangbing Fu et al.
Organic letters, 20(8), 2399-2402 (2018-04-12)
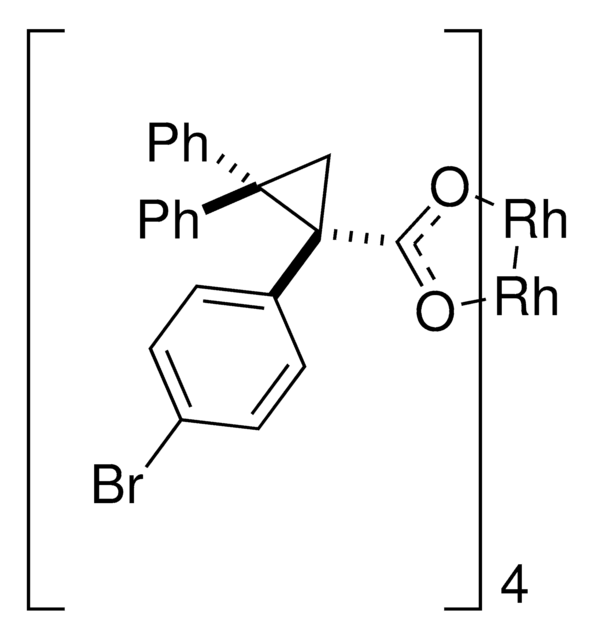
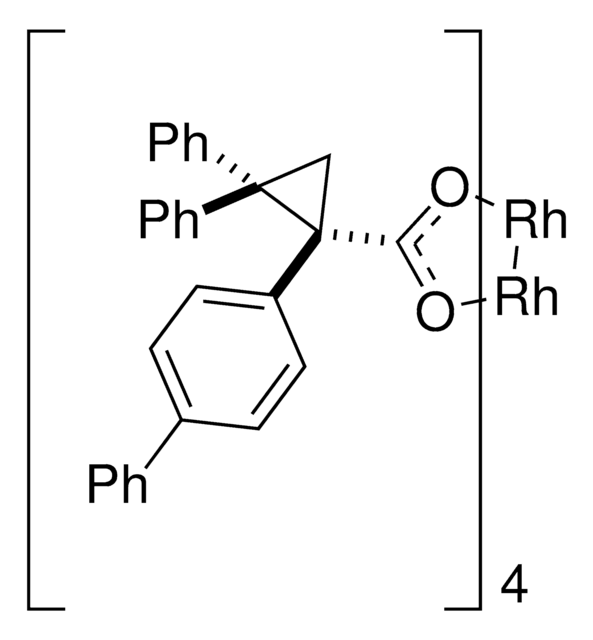
Rhodium(II)-catalyzed reactions between isopropyl acetate and trichloroethyl aryldiazoacetates result in the formation of oxirane intermediates that ring open under the reaction conditions to form tertiary alcohols. When the reaction is catalyzed by the dirhodium tetrakis(triarylcyclopropanecarboxylate) complex, Rh2( S-2-Cl,4-BrTPCP)4, the tertiary
Hengbin Wang et al.
Chemical science, 4(7), 2844-2850 (2013-09-21)
The rhodium-catalyzed reaction of electron-deficient alkenes with substituted aryldiazoacetates and vinyldiazoacetates results in highly stereoselective cyclopropanations. With adamantylglycine derived catalyst Rh2(S-TCPTAD)4, high asymmetric induction (up to 98% ee) can be obtained with a range of substrates. Computational studies suggest that
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.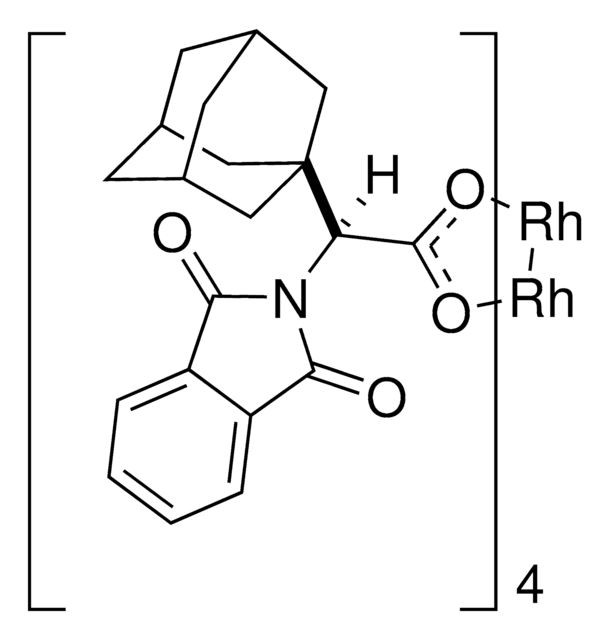



![Bis[Rhodium(α,α,α′,α′-tetramethyl-1,3-Benzoldipropionsäure)] 95%](/deepweb/assets/sigmaaldrich/product/structures/102/178/d1171a49-0358-406b-8b32-04324dbf9c02/640/d1171a49-0358-406b-8b32-04324dbf9c02.png)