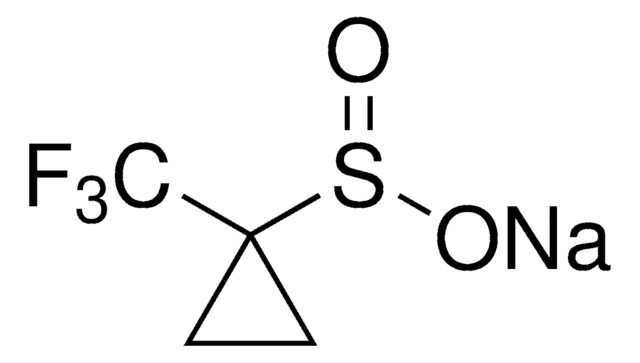
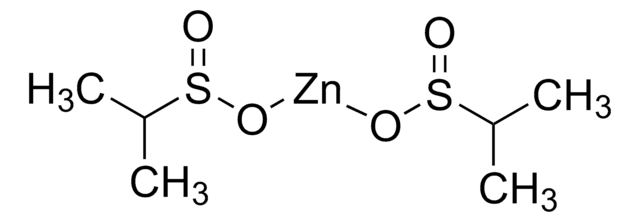
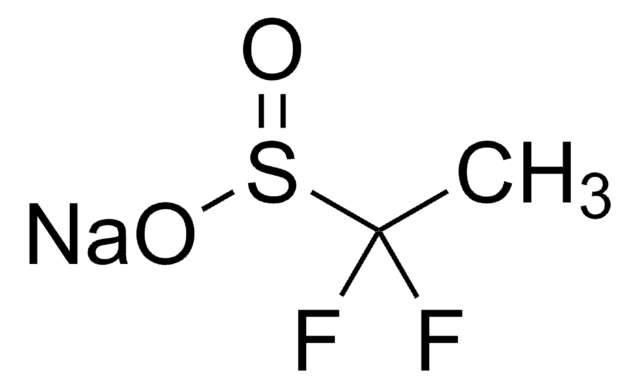
790796
Zinc benzylsulfinate
Synonym(e):
Zinc benzylsulfinate, Baran BNS Reagent
About This Item
Empfohlene Produkte
Form
solid
Eignung der Reaktion
reaction type: Alkylations
reaction type: C-C Bond Formation
reagent type: catalyst
reaction type: C-H Activation
reagent type: diversification reagent
mp (Schmelzpunkt)
56 °C (Decomposition)
Lagertemp.
2-8°C
SMILES String
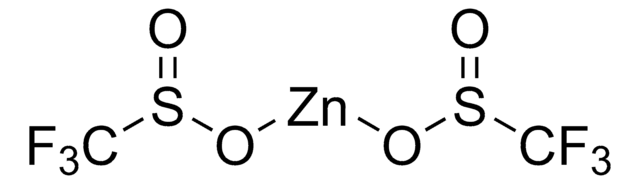
O=S(O[Zn]OS(CC1=CC=CC=C1)=O)CC2=CC=CC=C2
InChI
1S/2C7H8O2S.Zn/c2*8-10(9)6-7-4-2-1-3-5-7;/h2*1-5H,6H2,(H,8,9);/q;;+2/p-2
InChIKey
AHSIXHPQMGLVIG-UHFFFAOYSA-L
Anwendung
Practical and Innate Carbon-Hydrogen Functionalization of Heterocycles
Learn More at the Professor and Product Portal of Professor Phil S. Baran.
Sonstige Hinweise
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Artikel
The synthesis of heteroaromatic and aromatic compounds is at the heart of the chemical industry. The ever-growing demand for new chemical entities, coupled with dwindling resources and time constraints allotted to any given research project, a rapid way to diversify (hetero)aromatic scaffolds is needed.
Verwandter Inhalt
The synthesis of heteroaromatic and aromatic compounds is at the heart of the chemical industry. The ever-growing demand for new chemical entities, coupled with dwindling resources and time constraints allotted to any given research project, a rapid way to diversify (hetero)aromatic scaffolds is needed.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.