Wichtige Dokumente
764639
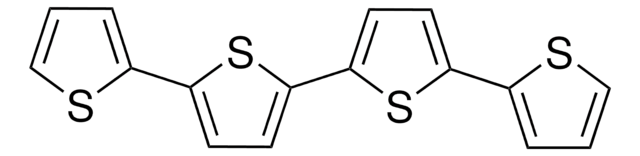
5,5′′′-Bis(tridecafluorohexyl)-2,2′:5′,2 ′′:5′′,2′′′-quaterthiophene
Synonym(e):
α,ω-Diperfluorohexyl-quarterthiophene, DFH-4T
About This Item
Empfohlene Produkte
Form
solid
Qualitätsniveau
mp (Schmelzpunkt)
205-210 °C
Halbleitereigenschaften
N-type (mobility≤0.64 cm2/V·s)
SMILES String
FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)c1ccc(s1)-c2ccc(s2)-c3ccc(s3)-c4ccc(s4)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C28H8F26S4/c29-17(30,19(33,34)21(37,38)23(41,42)25(45,46)27(49,50)51)15-7-5-13(57-15)11-3-1-9(55-11)10-2-4-12(56-10)14-6-8-16(58-14)18(31,32)20(35,36)22(39,40)24(43,44)26(47,48)28(52,53)54/h1-8H
InChIKey
UBMTYFFPSPVBSP-UHFFFAOYSA-N
Allgemeine Beschreibung
Anwendung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Artikel
Intrinsically stretchable active layers for organic field-effect transistors (OFET) are discussed. Polymer structural modification & post-polymerization modifications are 2 methods to achieve this.
Fabrication procedure of organic field effect transistor device using a soluble pentacene precursor.
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.
There is widespread demand for thin, lightweight, and flexible electronic devices such as displays, sensors, actuators, and radio-frequency identification tags (RFIDs). Flexibility is necessary for scalability, portability, and mechanical robustness.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.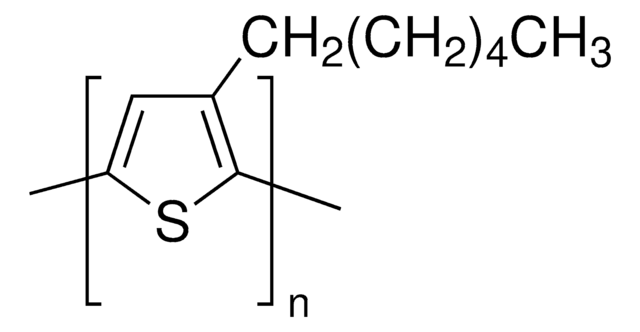
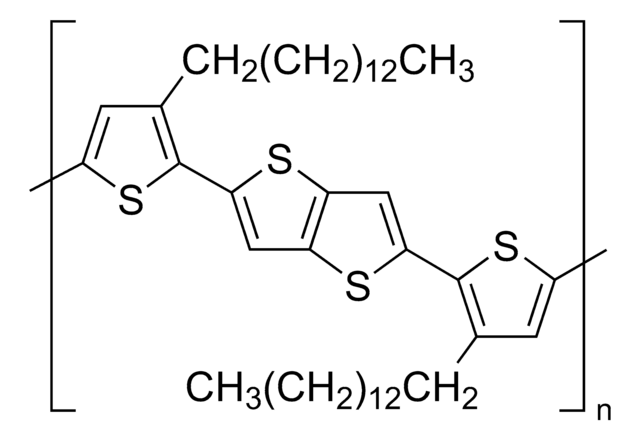
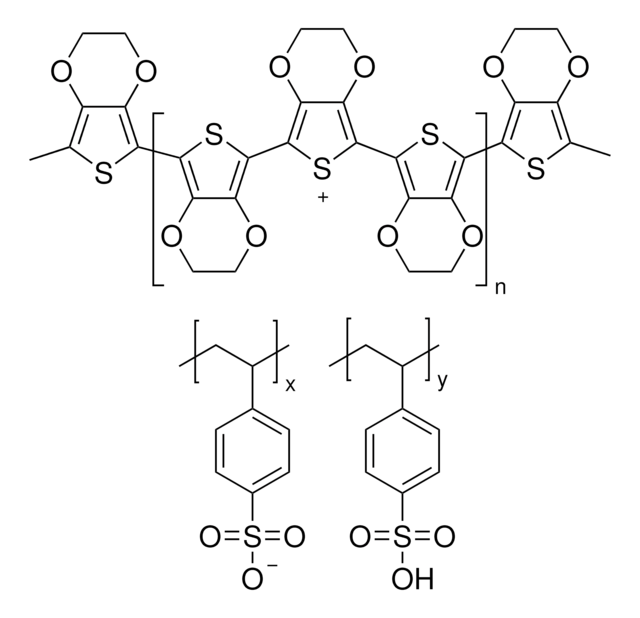
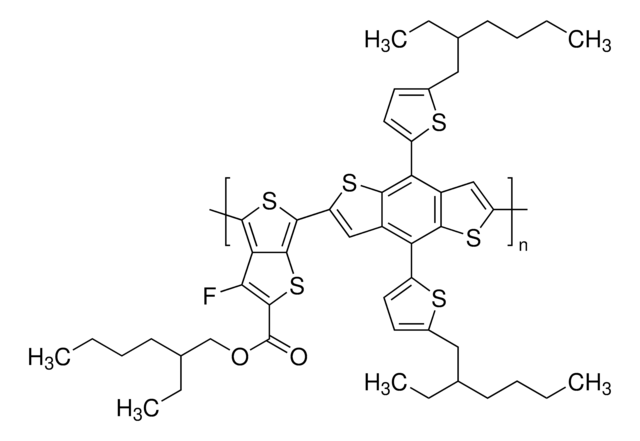
![Poly-[bis-(4-phenyl)-(2,4,6-trimethylphenyl)-amin] a poly(triaryl amine) semiconductor](/deepweb/assets/sigmaaldrich/product/structures/122/933/c34a34ab-284f-4890-adb8-126247a91d9b/640/c34a34ab-284f-4890-adb8-126247a91d9b.png)