533076
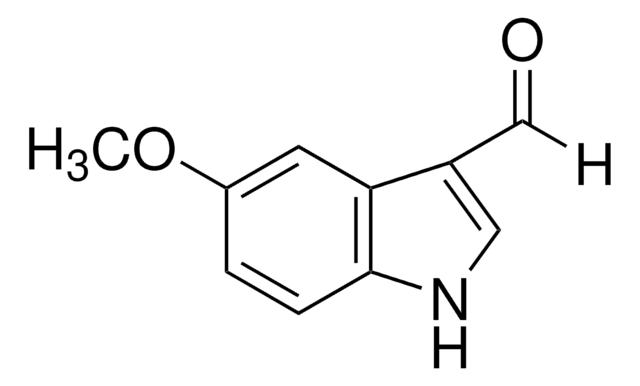
5-Chlorindol-3-carboxaldehyd
98%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C9H6ClNO
CAS-Nummer:
Molekulargewicht:
179.60
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
Empfohlene Produkte
Assay
98%
mp (Schmelzpunkt)
213-216 °C (lit.)
Funktionelle Gruppe
aldehyde
chloro
SMILES String
Clc1ccc2[nH]cc(C=O)c2c1
InChI
1S/C9H6ClNO/c10-7-1-2-9-8(3-7)6(5-12)4-11-9/h1-5,11H
InChIKey
YXEXOIGXNYITQH-UHFFFAOYSA-N
Allgemeine Beschreibung
5-Chloroindole-3-carboxaldehyde, also known as 5-chloro-1H-indole-3-carboxaldehyde, is an indole derivative.
Anwendung
5-Chloroindole-3-carboxaldehyde (5-Chloro-1H-indole-3-carboxaldehyde) may be used in the preparation of:
It may also be used in the preparation of the following hydrazone derivatives:
- 5-chloroindole-3-carboxaldehyde isonicotinoyl hydrazine
- 2′-[(5-chloro-1H-indol-3-yl)methylene]-2-(1H-indol-3-yl)acetohydrazide
- 5-chloro-3-(2,2-dibromovinyl)-1-(2-trimethylsilylethoxymethyl)indole
It may also be used in the preparation of the following hydrazone derivatives:
- 5-chloroindole-3-carboxaldehyde 3-chlorobenzoylhydrazone
- 5-chloroindole-3-carboxaldehyde 4-nitrobenzoylhydrazone
- 5-chloroindole-3-carboxaldehyde 3-methylbenzoylhydrazone
- 5-chloroindole-3-carboxaldehyde 4-methylbenzoylhydrazone
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Electrochemical behavior of indole-3-carboxaldehyde izonicotinoyl hydrazones: discussion on possible biological behavior
Shirinzadeh H, et al.
Combinatorial Chemistry & High Throughput Screening, 13(7), 619-627 (2010)
Tandem Suzuki-Miyaura cross-coupling/dehydrobromination of 1, 1-dibromoalkenes to alkynes with a cyclobutene-1, 2-diylbis (imidazolium) salt as catalyst precursor.
Rahimi A and Schmidt A.
Synthesis, 2010(15), 2621-2625 (2010)
2?-[(5-Chloro-1H-indol-3-yl) methylene]-2-(1H-indol-3-yl) acetohydrazide.
Ali HM, et al.
Acta Crystallographica Section E, Structure Reports Online, 63(4), o1807-o1808 (2007)
Kamaleddin Haj Mohammad Ebrahim Tehrani et al.
Iranian journal of pharmaceutical research : IJPR, 14(4), 1077-1086 (2015-12-15)
A series of indole-based aryl(aroyl)hydrazone analogs of antiplatelet indole-3-carboxaldehyde phenylhydrazone were synthesized by the Schiff base formation reaction and their antiplatelet activity was assessed using human platelet rich plasma. The platelet concentrate was obtained using a two-step centrifugation protocol and
Ming-Zhi Zhang et al.
European journal of medicinal chemistry, 92, 776-783 (2015-01-31)
Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Based on the methods developed for the synthesis of
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.