Alle Fotos(1)
Wichtige Dokumente
532223
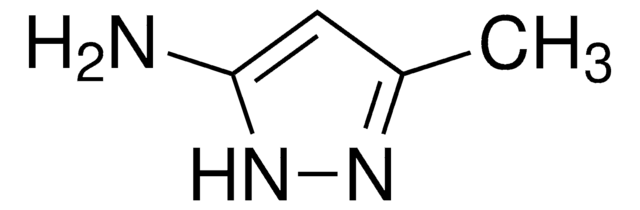
5-Amino-1,3-dimethylpyrazol
97%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C5H9N3
CAS-Nummer:
Molekulargewicht:
111.15
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
97%
mp (Schmelzpunkt)
65-69 °C (lit.)
SMILES String
Cc1cc(N)n(C)n1
InChI
1S/C5H9N3/c1-4-3-5(6)8(2)7-4/h3H,6H2,1-2H3
InChIKey
ZFDGMMZLXSFNFU-UHFFFAOYSA-N
Allgemeine Beschreibung
5-Amino-1,3-dimethylpyrazole undergoes cyclocondensation with ethyl acetoacetate to form the corresponding tetrahydropyrazolopyridine derivatives.
Anwendung
5-Amino-1,3-dimethylpyrazole may be used in the preparation of:
- 5-benzamido-1,3-dimethylpyrazole
- diethyl 2-{[(1,3-dimethyl-1H-pyrazol-5-yl)amino]methylene}malonate
- 5-amino-1,3-dimethyl-4-phthalidylpyrazole
- (E)-N-(3,7-dimethylocta-2,6-dienyl)-1,3-dimethyl-1H-pyrazol-5-amine analog(LQFM002)
- 4-isopropyl-1,3-dimethyl-1H-pyrazolo[3,4-b]pyridin-6-ol
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
190.4 °F - closed cup
Flammpunkt (°C)
88 °C - closed cup
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Hiroshi Ochiai et al.
Chemical & pharmaceutical bulletin, 52(9), 1098-1104 (2004-09-02)
A series of 4-anilinopyrazolopyridine derivatives were synthesized and biologically evaluated as inhibitors of phosphodiesterase (PDE4). Chemical modification of 3, a structurally new chemical lead that was found in our in-house library, was focused on 1- and 3-substituents. Full details of
The cyclocondensation of 5?amino?1,3?dimethylpyrazole with ethyl acetoacetate. Synthesis of isomeric pyrazolopyridones.
Ratajczyk JD and Swett LR.
Journal of Heterocyclic Chemistry, 12(3), 517-522 (1975)
1,3-Oxazines and related compounds. II.1) Ring contraction reaction of 1, 3-oxazin-4-one derivatives into 1,2,4-triazoles and pyrazoles.
Yamamoto, et al.
Chemical & Pharmaceutical Bulletin, 26(6), 1825-1831 (1978)
The reaction of o?phthalaldchydic acid with 5?amino?1,3?dimethylpyrazole.
Swett LR and Aynilian GH.
Journal of Heterocyclic Chemistry, 12(6), 1135-1136 (1975)
E A Costa et al.
Life sciences, 92(3), 237-244 (2013-01-09)
The current study describes the synthesis and pharmacological evaluation of (E)-N-(3,7-dimethylocta-2,6-dienyl)-1,3-dimethyl-1H-pyrazol-5-amine (LQFM002), a compound originally designed through a molecular simplification strategy from 4-nerolidylcatechol. LQFM002 was evaluated for preservation of the PLA(2) enzyme inhibitory effects of the lead compound, 4-nerolidylcatechol, using
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.