Alle Fotos(4)
Wichtige Dokumente
521418
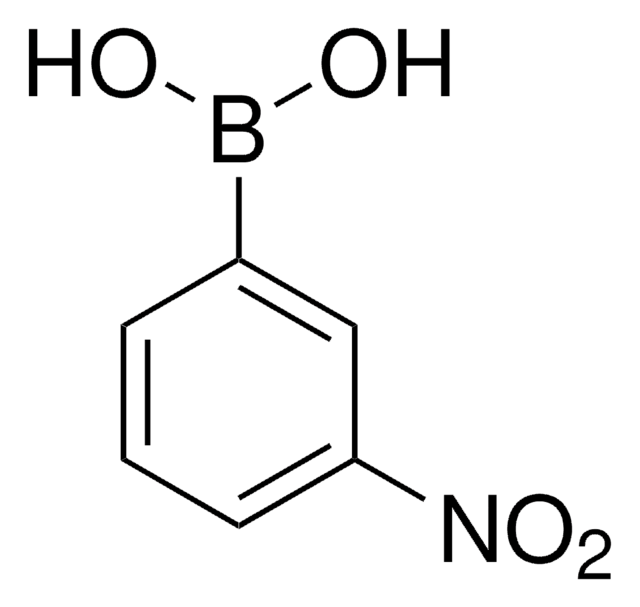
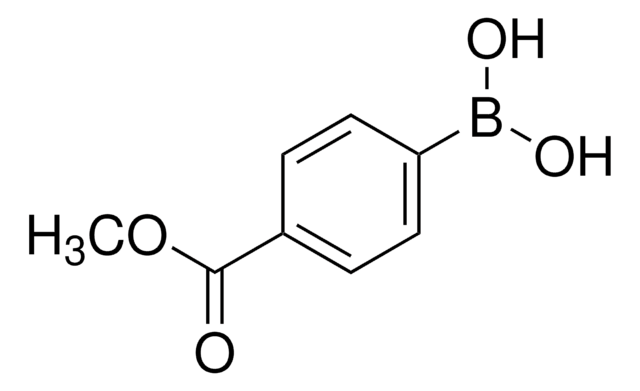
4-Cyanophenylborsäure
≥95%
Synonym(e):
(p-Cyanophenyl)boronic acid, 4-Cyanobenzeneboronic acid, 4-Cyanophenylboric acid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(4)
About This Item
Lineare Formel:
NCC6H4B(OH)2
CAS-Nummer:
Molekulargewicht:
146.94
MDL-Nummer:
UNSPSC-Code:
12352103
PubChem Substanz-ID:
NACRES:
NA.22
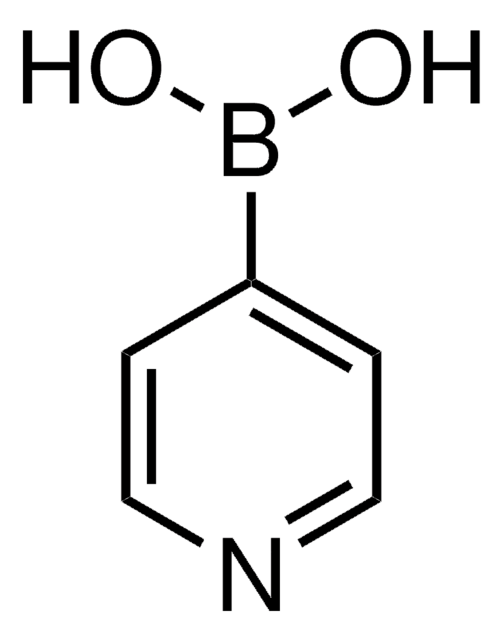
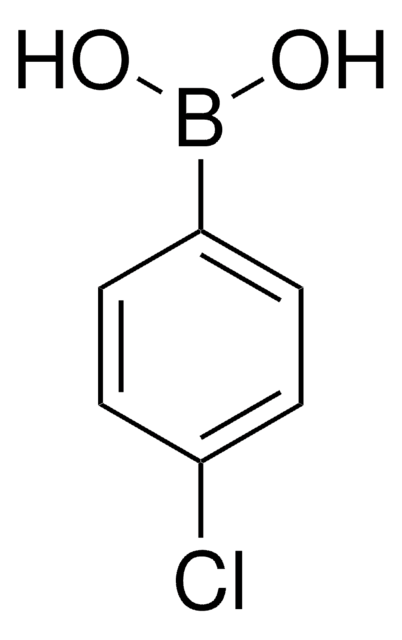
Empfohlene Produkte
Qualitätsniveau
Assay
≥95%
mp (Schmelzpunkt)
>350 °C (lit.)
Funktionelle Gruppe
nitrile
SMILES String
OB(O)c1ccc(cc1)C#N
InChI
1S/C7H6BNO2/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4,10-11H
InChIKey
CEBAHYWORUOILU-UHFFFAOYSA-N
Anwendung
4-Cyanophenylboronic acid can be used as a reactant in:
It can also be used to prepare:
- Palladium-catalyzed Suzuki-Miyaura cross-coupling in water.
- Ruthenium catalyzed direct arylation of benzylic sp3 carbons of acyclic amines with arylboronates.
- Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.
- Ferric perchlorate-promoted reaction of fullerenes with various arylboronic acids to give fullerenyl boronic esters.
- Phosphine-free Suzuki-Miyaura cross-coupling.
- Palladacycles as effective catalysts for multicomponent reaction with allylpalladium-intermediates.
- Chan-Lam-type Cu-catalyzed S-arylation of thiols.
- Regioselective cross-coupling reactions under modfied Suzuki and Still cross-coupling reactions with copper catalysis.
- Metal-free biaryl coupling reaction in the presence of dimethyl carbonate as a solvent.
- Suzuki-type cross-coupling reaction with pentavalent triarylantimony diacetates in the absence of a base.
It can also be used to prepare:
- Himbacine analogs as thrombin receptor antagonists and potential antiplatelet agents.
- Trisulfonated calixarene upper-rim sulfonamido and their complexation with trimethyllysine epigenetic mark.
- Antimalarial compounds via Suzuki cross-coupling.
- Deoxyuridine derivatives.
Reactant involved in:
Precursor in the synthesis of inhibitors such as:
- Oxidative hydroxylation
- Trifluoromethylation
- 1,4-Addition reactions
Precursor in the synthesis of inhibitors such as:
- Tpl2 kinase inhibitors
- P2X7 antagonists used in the treatment of pain
Sonstige Hinweise
Contains varying amounts of anhydride
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Discovery of nor-seco himbacine analogs as thrombin receptor antagonists
Chelliah, M. V.; et al.
Bioorganic & Medicinal Chemistry, 22, 2544-2549 (2012)
Mohamed A Ismail et al.
Journal of medicinal chemistry, 47(14), 3658-3664 (2004-06-25)
2-[5-(4-Amidinophenyl)-furan-2-yl]-5,6,7,8-tetrahydro-imidazo[1,2-a]pyridine-6-carboxamidine acetate salt (7) was synthesized from 2-[5-(4-cyanophenyl)-furan-2-yl]-imidazo[1,2-a]pyridine-6-carbonitrile (4a), through the bis-O-acetoxyamidoxime followed by hydrogenation in glacial acetic acid. Compound 4a was obtained in four steps starting with two successive brominations of 2-acetylfuran first with N-bromosuccinimide, and second with bromine
A mild and efficient new synthesis of aryl sulfones from boronic acids and sulfinic acid salts
Christian Beaulieu, et al.
Tetrahedron Letters, 45, 3233-3236 (2004)
Palladacycles: Effective catalysts for a multicomponent reaction with allylpalladium(II)-intermediates
Shiota, A.; Malinakova, H. C.
Journal of Organometallic Chemistry, 704, 9-16 (2012)
Yassir Younis et al.
Journal of medicinal chemistry, 55(7), 3479-3487 (2012-03-07)
A novel class of orally active antimalarial 3,5-diaryl-2-aminopyridines has been identified from phenotypic whole cell high-throughput screening of a commercially available SoftFocus kinase library. The compounds were evaluated in vitro for their antiplasmodial activity against K1 (chloroquine and drug-resistant strain)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![[1,1′-Bis(diphenylphosphino)ferrocen]dichlorpalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)