Alle Fotos(2)
Wichtige Dokumente
515396
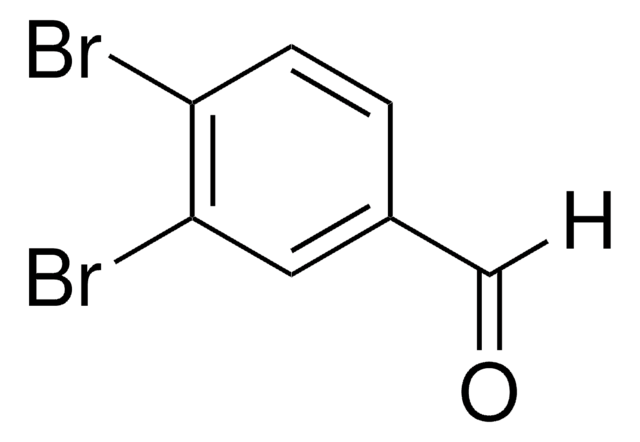
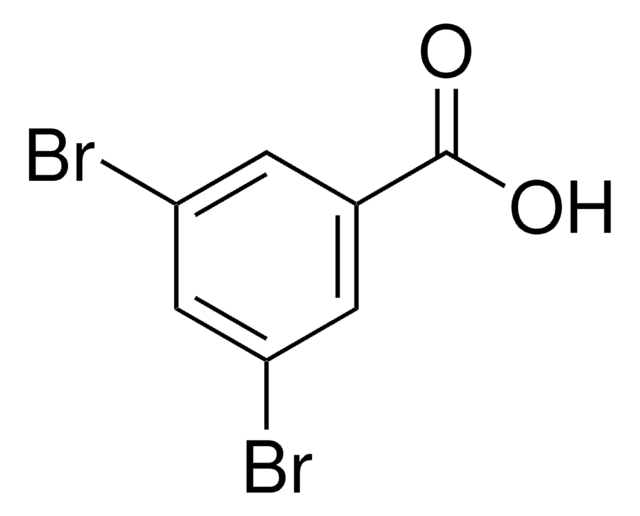
3,5-Dibrombenzaldehyd
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Lineare Formel:
Br2C6H3CHO
CAS-Nummer:
Molekulargewicht:
263.91
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
mp (Schmelzpunkt)
84-88 °C (lit.)
Funktionelle Gruppe
aldehyde
bromo
SMILES String
Brc1cc(Br)cc(C=O)c1
InChI
1S/C7H4Br2O/c8-6-1-5(4-10)2-7(9)3-6/h1-4H
InChIKey
ZLDMZIXUGCGKMB-UHFFFAOYSA-N
Verwandte Kategorien
Anwendung
Reactant involved in:
- Suzuki-Miyaura cross-coupling reactions
- Synthesis of blue fluorescent dye derivatives for organic light emitting diodes
- Sharpless kinetic resolution for the formation of Baylis-Hillman enal adducts
- Synthesis of podophyllotoxin mimetic pyridopyrazoles as anticancer agents
- Allylic alkylation
- Synthesis of C2-symmetric biphosphine ligand I
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Skin Corr. 1B
Lagerklassenschlüssel
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Jakub Saadi et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(12), 3842-3845 (2013-02-21)
Double-action haloketones: A super silyl group enabled the first highly diastereoselective Mukaiyama aldol reactions of α-chloro- and α-fluoroketones with a wide range of aldehydes, providing anti-β-siloxy-α-haloketones. This process is compatible with one-pot double-aldol methodology and allows for rapid access to
Synthesis, crystal structures, and antibacterial activity of a series of hydrazone compounds derived from 4-methylbenzohydrazide.
Lei Y, et al.
Journal of the Chilean Chemical Society, 60(2), 2961-2965 (2015)
Punit P Seth et al.
Bioorganic & medicinal chemistry letters, 14(22), 5569-5572 (2004-10-16)
The preparation and evaluation of novel aryl urea analogs as broad-spectrum antibacterial agents is described. Numerous compounds showed low micromolar minimum inhibitory concentrations (MIC) against both Gram-positive and Gram-negative bacteria. Selected analogs also exhibited in vivo efficacy in a lethal
Synthesis and luminescence characteristics of conjugated dendrimers with 2, 4, 6-triaryl-1, 3, 5-triazine periphery.
Kim CK, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 44(1), 254-263 (2006)
Conformational Behavior of Conjugated Polymers With Oligo (phenylene vinylene) Side Chains.
Peeter H and Koeckelberghs G.
Macromolecular Chemistry and Physics, 214(5), 538-546 (2013)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.