Wichtige Dokumente
394467
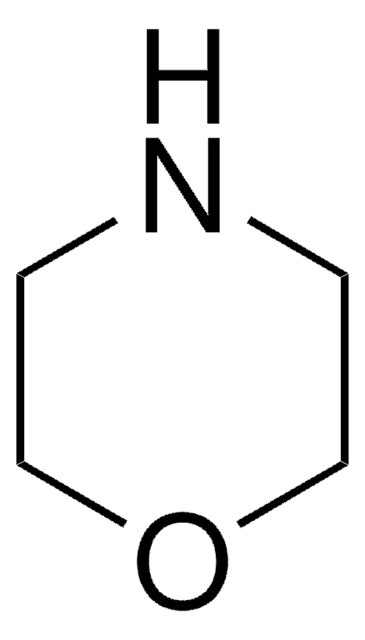
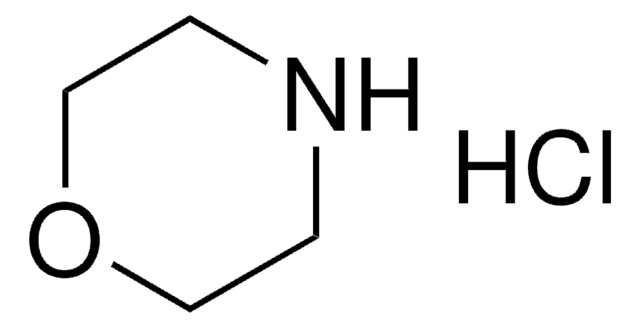
Morpholin
purified by redistillation, ≥99.5%
Synonym(e):
Tetrahydro-1,4-oxazin
About This Item
Empfohlene Produkte
Dampfdichte
3 (vs air)
Dampfdruck
31 mmHg ( 38 °C)
7 mmHg ( 20 °C)
Assay
≥99.5%
Form
liquid
Selbstzündungstemp.
590 °F
Aufgereinigt durch
redistillation
Expl.-Gr.
10.8 %
Brechungsindex
n20/D 1.454 (lit.)
bp
129 °C (lit.)
129 °C
mp (Schmelzpunkt)
−7-−5 °C (lit.)
Löslichkeit
water: miscible
Dichte
0.996 g/mL at 25 °C (lit.)
Funktionelle Gruppe
ether
SMILES String
C1COCCN1
InChI
1S/C4H9NO/c1-3-6-4-2-5-1/h5H,1-4H2
InChIKey
YNAVUWVOSKDBBP-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
It may be used in the following studies:
- As a reactant in the synthesis of 1,3-dihydro-1-hydroxy-3-morpholin-4-yl-2,1-benzoxaborole by reacting with o-formylphenylboronic acid.
- As a corrosion inhibitor and to maintain basic pH in boiler feed water.
- As a reactant in the bis(β-ketoenolates)nickel(II) adducts of morpholine.
- As one of the reagent used in the colorimetric quantitative determination of C-2 unsubstituted phenothiazine derivatives.
- As a reactant in the quantitative determination of α,β-unsaturated compounds.
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Repr. 2 - Skin Corr. 1B
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 1
Flammpunkt (°F)
87.8 °F - closed cup
Flammpunkt (°C)
31 °C - closed cup
Persönliche Schutzausrüstung
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.