Alle Fotos(1)
Wichtige Dokumente
357839
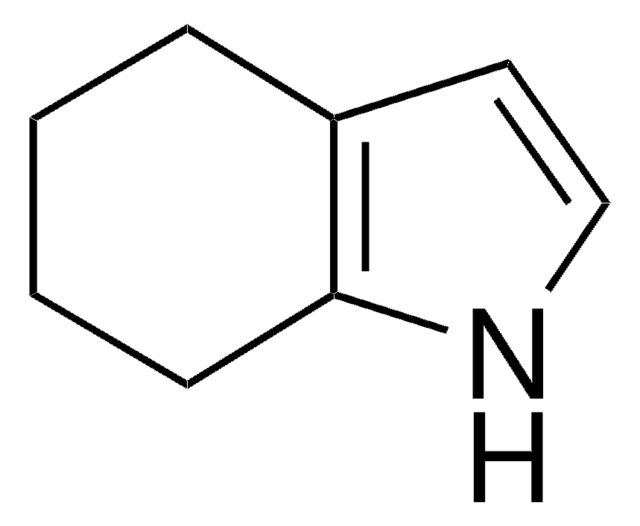
1,5,6,7-Tetrahydro-4H-indol-4-on
98%
Synonym(e):
4,5,6,7-Tetrahydro-4-oxoindole, NSC 131681
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C8H9NO
CAS-Nummer:
Molekulargewicht:
135.16
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
mp (Schmelzpunkt)
188-190 °C (lit.)
Funktionelle Gruppe
ketone
SMILES String
O=C1CCCc2[nH]ccc12
InChI
1S/C8H9NO/c10-8-3-1-2-7-6(8)4-5-9-7/h4-5,9H,1-3H2
InChIKey
KASJZXHXXNEULX-UHFFFAOYSA-N
Allgemeine Beschreibung
Iodination of 1,5,6,7-tetrahydro-4H-indol-4-one using 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) yields α-iodo derivative as the main product.
Anwendung
1,5,6,7-Tetrahydro-4H-indol-4-one may be used in the preparation of:
- 4-oxo-4,5,6,7-tetrahydro-1H-indole-2-carbonitrile
- methyl 2-(4-oxo-4,5,6,7-tetrahydro-1H-indol-1-yl)acrylate
- 3-(4-oxo-4,5,6,7-tetrahydro-1H-indol-1-yl)propanenitrile
- potent and orally active 5-HT1A agonists, (R)-(+)- and (S)-(-)-1-formyl-6,7,8,9-tetrahydro-N,N-dipropyl-3H-benz[e]indol-8-amines
- Reactant in synthesis of psammopemmin A as antitumor agent
- Reactant in synthesis of a 1,3,4,5-tetrahydrobenzindole β-ketoesters and tricyclic tetrahydrobenzindoles via C-H insertion reactions
- Reactant in preparation of tricyclic indole and dihydroindole derivatives as inhibitors of guanylate cyclase
- Reactant in preparation of condensed pyrroloindoles via Pd-catalyzed intramolecular C-H bond functionalization of (halobenzyl)pyrroles
- Reactant in enantioselective preparation of arylalkenyl indoles via asymmetric C-H insertion of rhodium carbenoids followed by Cope rearrangement-elimination
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
DNA binders: 1. evaluation of DNA-interactive ability, design, and synthesis of novel intercalating agents
Sechi M, et al.
Letters in Drug Design & Discovery, 6(1), 56-62 (2009)
Direct α-iodination of aryl alkyl ketones by elemental iodine activated by 1-chloromethyl-4-fluoro-1, 4-diazoniabicyclo [2.2. 2] octane bis (tetrafluoroborate).
Jereb M, et al.
Synthesis, 06, 0853-0858 (2003)
Synthesis of pyrrolo [1, 2-a] indole-1, 8 (5H)-diones as new synthons for developing novel tricyclic compounds of pharmaceutical interest.
Sechi M, et al.
ARKIVOC (Gainesville, FL, United States), 5, 97-106 (2004)
Synthesis of (R)-and (S)-1-formyl-6, 7, 8, 9-tetrahydro-N, N-(dipropyl)-3H-benz [e] indol-8-amines: potent and orally active 5-HT1A receptor agonists.
Lin C-H, et al.
Journal of Heterocyclic Chemistry, 31(1), 129-139 (1994)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.