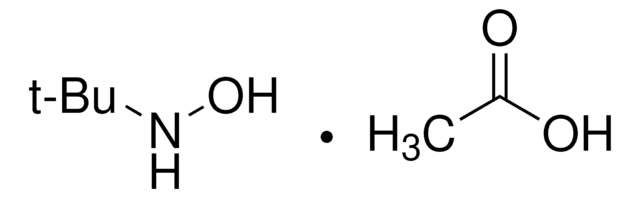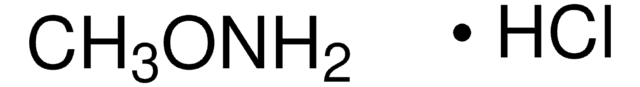Alle Fotos(3)
Wichtige Dokumente
221457
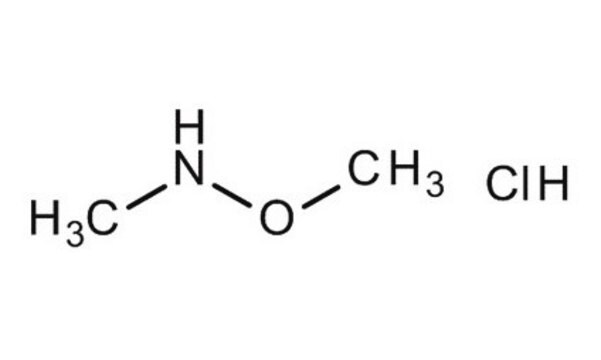
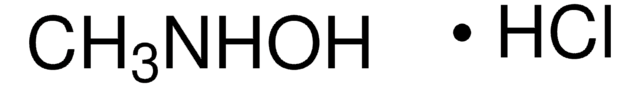
N,N-Dimethylhydroxylamin -hydrochlorid
99%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Lineare Formel:
(CH3)2NOH · HCl
CAS-Nummer:
Molekulargewicht:
97.54
Beilstein:
3905683
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
99%
Form
solid
mp (Schmelzpunkt)
107-109 °C (lit.)
Funktionelle Gruppe
amine
SMILES String
Cl[H].CN(C)O
InChI
1S/C2H7NO.ClH/c1-3(2)4;/h4H,1-2H3;1H
InChIKey
HWWVAHCWJLGKLW-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
N,N-Dimethylhydroxylamine hydrochloride is an organic compound often used as an organocatalyst to increase Morita-Baylis-Hillman reaction rate by reducing the activation energy of the rate-limiting aldol step.
Anwendung
N,N-Dimethylhydroxylamine hydrochloride was used in the synthesis of 4,4-dimethyl-2,5,5-triphenyl-l.3-dioxa-4-azonia-2-bora-5-boratacyclopentane. It was also used as a polymer-chain terminator.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Structural studies of organoboron compounds. XVI. Preparation and crystal and molecular structures of 4, 4-dimethyl-2, 5, 5-triphenyl-1, 3-dioxa-4-azonia-2-bora-5-boratacyclopentane and 4, 4, 5, 5-tetramethyl-2, 2-diphenyl-1, 3-dioxa-4-azonia-2-boratacyclopentane.
Canadian Journal of Chemistry, 62(5), 838-844 (1984)
Hana Popelkova et al.
Photosynthesis research, 110(2), 111-121 (2011-11-02)
The photosystem II (PSII) manganese-stabilizing protein (PsbO) is known to be the essential PSII extrinsic subunit for stabilization and retention of the Mn and Cl(-) cofactors in the oxygen evolving complex (OEC) of PSII, but its function relative to Ca(2+)
A R Tunoori et al.
Organic letters, 2(25), 4091-4093 (2000-12-12)
[structure] The reagent [bis(2-methoxyethyl)amino]sulfur trifluoride (Deoxo-Fluor reagent) converts carboxylic acids to the corresponding acid fluorides, which then react with N,N-dimethylhydroxylamine to give the corresponding Weinreb amides in high yields. The reaction proceeds without racemization when optically active acids are used
K Stolze et al.
Free radical research communications, 8(2), 123-131 (1990-01-01)
Nitroxide radicals have been detected in the methemoglobin formation reaction between oxyhemoglobin and the substituted hydroxylamine compounds, N-methylhydroxylamine and N,N-dimethylhydroxylamine, by ESR spectroscopy. The stability of these nitroxide radicals was considerably higher than that of the NH2O. radical derived from
J Taira et al.
Biochimica et biophysica acta, 1336(3), 502-508 (1997-11-21)
Hydroxylamine (HA), which is a natural product of mammalian cells, has been shown to possess vasodilatory properties in several model systems. In this study, HA and methyl-substituted hydroxylamines, N-methylhydroxylamine (NMHA) and N,N-dimethylhydroxylamine (NDMHA), have been tested for their ability to
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.