Wichtige Dokumente
168521
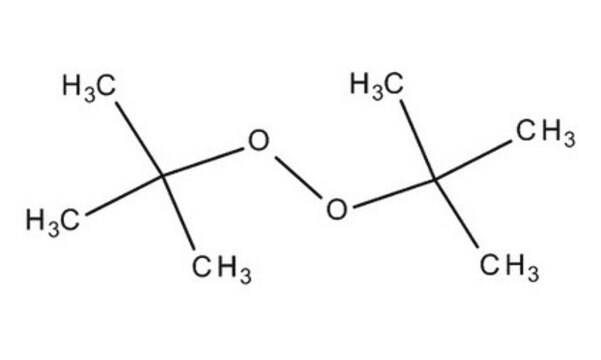
Luperox® DI, tert-Butylperoxid
98%
Synonym(e):
Di-tert.-butylperoxid, tert.-Butylperoxid
About This Item
Empfohlene Produkte
Dampfdruck
40 mmHg ( 20 °C)
Qualitätsniveau
Assay
98%
Form
liquid
Eignung der Reaktion
reagent type: oxidant
Brechungsindex
n20/D 1.3891 (lit.)
bp
109-110 °C (lit.)
Dichte
0.796 g/mL at 25 °C (lit.)
Funktionelle Gruppe
peroxide
Lagertemp.
2-8°C
SMILES String
CC(C)(C)OOC(C)(C)C
InChI
1S/C8H18O2/c1-7(2,3)9-10-8(4,5)6/h1-6H3
InChIKey
LSXWFXONGKSEMY-UHFFFAOYSA-N
Verwandte Kategorien
Anwendung
Rechtliche Hinweise
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Aquatic Chronic 3 - Flam. Liq. 2 - Muta. 2 - Org. Perox. E
Lagerklassenschlüssel
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 1
Flammpunkt (°F)
42.8 °F - closed cup
Flammpunkt (°C)
6 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 168521-1L | 4061838749901 |
| 168521-250ML | 4061838749918 |
| 168521-5ML | 4061838749925 |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.