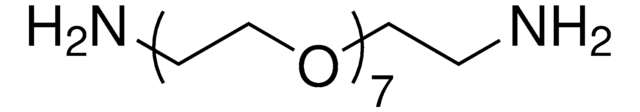14502
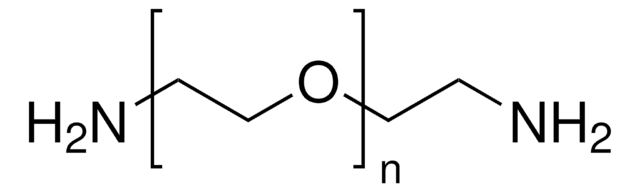
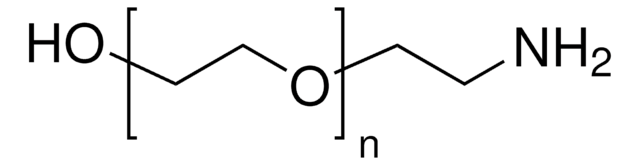
Poly(ethylenglycol)bis(amin)
Mw 3,000, carboxyl reactive, amine
Synonym(e):
O,O′-Bis-(2-aminoethyl)-polyethylenglykol, PEG-Diamin, Polyoxyethylenbis(amin)
About This Item
Empfohlene Produkte
product name
Poly(ethylenglycol)bis(amin), Mw 3,000
Mol-Gew.
Mw 3,000
Qualitätsniveau
Eignung der Reaktion
reagent type: cross-linking reagent
reactivity: carboxyl reactive
Ω-Ende
amine
α-Ende
amine
Polymerarchitektur
shape: linear
functionality: homobifunctional
InChI
1S/C6H16N2O2/c7-1-3-9-5-6-10-4-2-8/h1-8H2
InChIKey
IWBOPFCKHIJFMS-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Progress in biotechnology fields such as tissue engineering and drug delivery is accompanied by an increasing demand for diverse functional biomaterials. One class of biomaterials that has been the subject of intense research interest is hydrogels, because they closely mimic the natural environment of cells, both chemically and physically and therefore can be used as support to grow cells. This article specifically discusses poly(ethylene glycol) (PEG) hydrogels, which are good for biological applications because they do not generally elicit an immune response. PEGs offer a readily available, easy to modify polymer for widespread use in hydrogel fabrication, including 2D and 3D scaffold for tissue culture. The degradable linkages also enable a variety of applications for release of therapeutic agents.
Devising biomaterial scaffolds that are capable of recapitulating critical aspects of the complex extracellular nature of living tissues in a threedimensional (3D) fashion is a challenging requirement in the field of tissue engineering and regenerative medicine.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.