Alle Fotos(1)
Wichtige Dokumente
107425
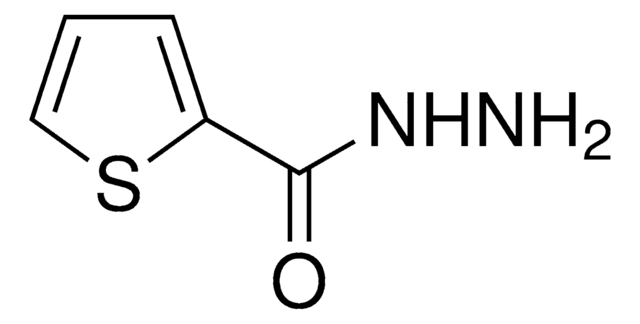
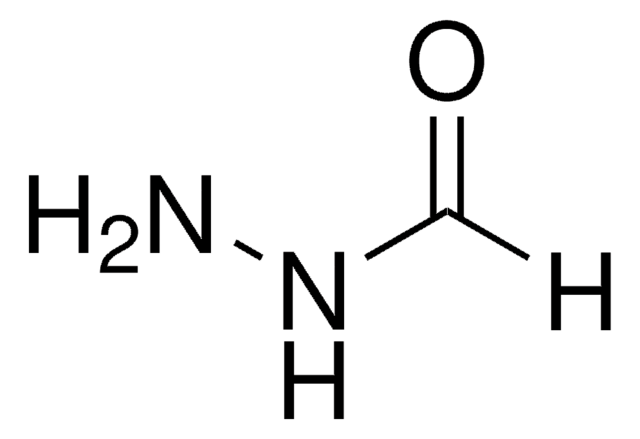
Nicotinsäurehydrazid
97%
Synonym(e):
Nicotinsäurehydrazid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C6H7N3O
CAS-Nummer:
Molekulargewicht:
137.14
Beilstein:
119299
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
97%
mp (Schmelzpunkt)
159-161 °C (lit.)
Löslichkeit
H2O: soluble 50 mg/mL
Funktionelle Gruppe
amine
hydrazine
SMILES String
NNC(=O)c1cccnc1
InChI
1S/C6H7N3O/c7-9-6(10)5-2-1-3-8-4-5/h1-4H,7H2,(H,9,10)
InChIKey
KFUSANSHCADHNJ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Nicotinic hydrazide is a heterocyclic compound that can be used to synthesize Schiff bases.
Anwendung
Nicotinic hydrazide was used in hydrazone library formation. It was used to study the oxidation of isonicotinic acid hydrazide (isoniazid) by horseradish peroxidase.
Biochem./physiol. Wirkung
Nicotinic hydrazide is an inhibitor of peroxidase enzyme. It forms solid metal complexes having strong biological activity.
Angaben zur Herstellung
Nicotinic hydrazide dissolves in water at a concentration of 50 mg/ml to form a clear, colourless solution.
Signalwort
Warning
H-Sätze
P-Sätze
Gefahreneinstufungen
Eye Irrit. 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
V Goral et al.
Proceedings of the National Academy of Sciences of the United States of America, 98(4), 1347-1352 (2001-02-15)
Dynamic combinatorial libraries are mixtures of compounds that exist in a dynamic equilibrium and can be driven to compositional self adaptation via selective binding of a specific assembly of certain components to a molecular target. We present here an extension
Thermo-chemical behavior of solid nicotinic hydrazide metal complexes in correlation with their stoichiometry.
Sekkina MM and El-Azm MG.
Thermochimica Acta, 77(1), 211-218 (1984)
H A Shoeb et al.
Antimicrobial agents and chemotherapy, 27(3), 399-403 (1985-03-01)
Oxidation of isonicotinic acid hydrazide (isoniazid) by horseradish peroxidase at the expense of H2O2 yielded reactive species which were able to reduce nitroblue tetrazolium and bleach p-nitrosodimethylaniline. Nicotinic acid hydrazide oxidation did not cause these effects. At slightly alkaline pH
Alireza Moradi et al.
Archiv der Pharmazie, 343(9), 509-518 (2010-09-02)
A series of 2-phenoxynicotinic acid hydrazides were synthesized and evaluated for their analgesic and anti-inflammatory activities. Several compounds having an unsubstituted phenyl/4-pyridyl or C-4 methoxy substituent on the terminal phenyl ring showed moderate to high analgesic or anti-inflammatory activity in
[Tautomerism of 2-hydrazino-4-phenylthiazole<-->4-phenylthiazol-2-one hydrazone. Derivatives of acids. II. (4-phenyl-3-R-thiazol-2-ylidene) and beta-methyl-beta-(4-phenylthiazol-2-yl) hydrazides of picolinic, nicotinic, and isonicotinic acid].
L Bielak et al.
Annales Universitatis Mariae Curie-Sklodowska. Sectio D: Medicina, 44, 41-51 (1989-01-01)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.