442232-U
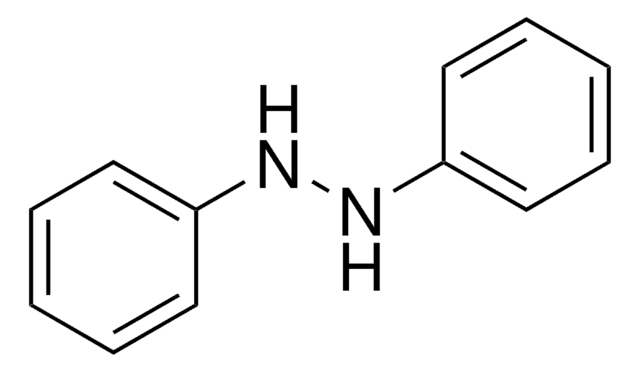
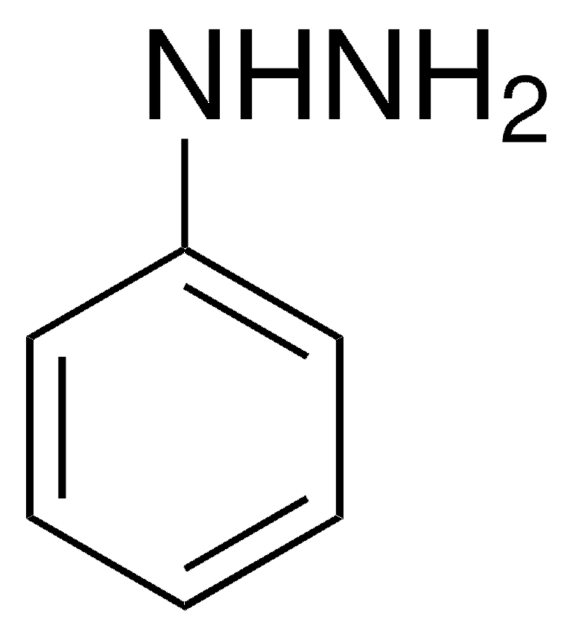
1,2-Diphenylhydrazine
analytical standard, ampule of 100 mg
Synonyme(s) :
Hydrazobenzene, N,N′-diphénylhydrazine
About This Item
Produits recommandés
Qualité
analytical standard
CofA (certificat d'analyse)
current certificate can be downloaded
Conditionnement
ampule of 100 mg
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Pf
123-126 °C (lit.)
Application(s)
cleaning products
cosmetics
environmental
food and beverages
personal care
Format
neat
Température de stockage
2-30°C
Chaîne SMILES
N(Nc1ccccc1)c2ccccc2
InChI
1S/C12H12N2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10,13-14H
Clé InChI
YBQZXXMEJHZYMB-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- Insertion reactions with organometallic tantalum complexes
- Reduction reactions catalyzed by titanium(III) trichloride yielding amines
- Studying the mechanism of hydrazobenzene rearrangement
- Reaction with N-heterocyclic stable silylene
- Synthesis of dimanganese amide hydrazide cluster complexes
- Iron-mediated hydrazine reductions yielding iron arylimide cubanes
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B
Code de la classe de stockage
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves, type P2 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique