SAB4200800
Anti-Collagen, Type X antibody, Mouse monoclonal
clone COL-10, purified from hybridoma cell culture
Synonyme(s) :
Anti-COL10A1
About This Item
Produits recommandés
Source biologique
mouse
Forme d'anticorps
purified from hybridoma cell culture
Type de produit anticorps
primary antibodies
Clone
COL-10, monoclonal
Forme
buffered aqueous solution
Poids mol.
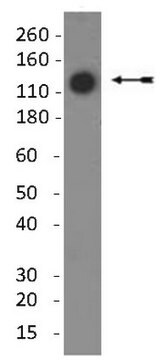
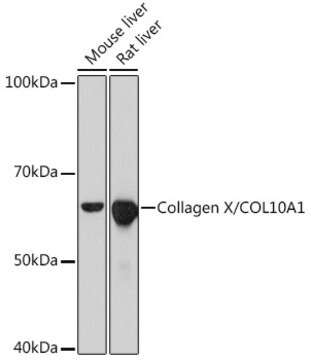
~60 kDa
Espèces réactives
deer, porcine, human
Conditionnement
antibody small pack of 25 μL
Concentration
~1 mg/mL
Technique(s)
immunoblotting: suitable
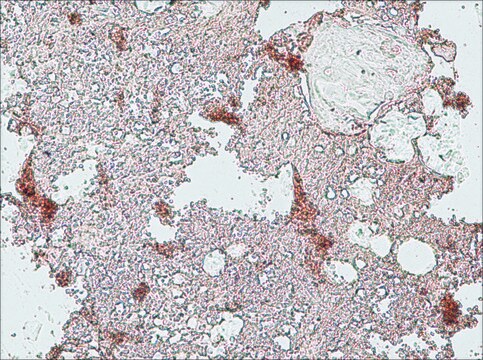
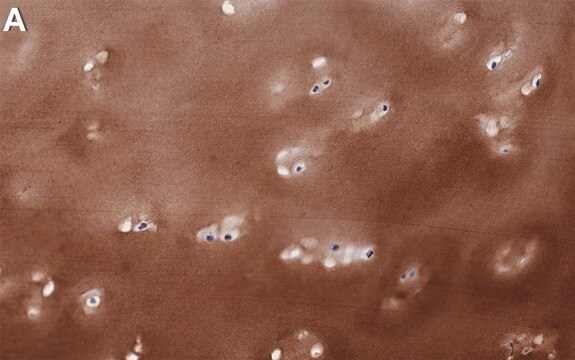
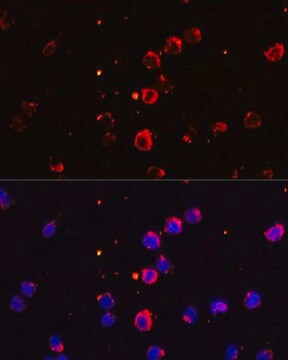
immunofluorescence: 5-10 μg/mL using human osteosarcoma SaOS-2 cells
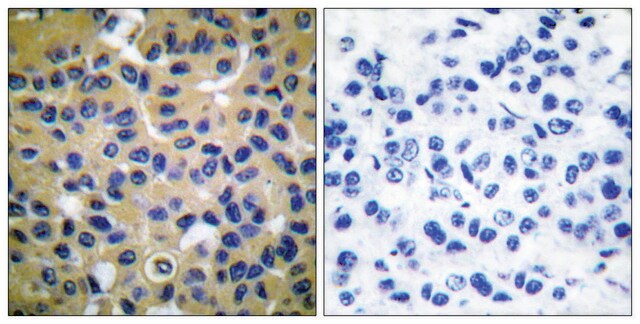
immunohistochemistry: suitable
Isotype
IgM
Numéro d'accès UniProt
Conditions d'expédition
dry ice
Température de stockage
−20°C
Modification post-traductionnelle de la cible
unmodified
Informations sur le gène
human ... COL10A1(1300)
Description générale
Type X collagen,also known as Collagen alpha-1(X) chain (COL10A1), is a product of hypertrophic chondrocytes. It shares a similar domain structure with type VIII collagen. In addition, both collagen types represent major components of hexagonal lattice structure, in which the collagen molecules link together by interactions involving the non-triple-helical end regions. Despite these similarities, a distinct tissue distribution has been found for these two molecules: type VIII collagen is distributed in various tissues, whereas type X is restricted to normal fetal hypertrophic cartilage in the growth zones of long bones, vertebrae and ribs and in adult (> 21 yr) thyroid cartilage. It is also found in bone fracture callus, osteoarthritic cartilage and chondrogenic neoplasms, and may be involved in cartilage mineralization. Type X collagen is non-fibrillar, but forms fine pericellular filaments in association with cartilage collagen. It interacts with matrix proteins, such as connexin V, chondrocalcein, collagen II and proteoglycans, as well as with Ca2+ . Mutations in this gene are associated with schmid metaphyseal chondroplasia (MCDS).
The development of antibodies against collagens has provided a powerful method for examining the distribution of these connective tissue proteins and for investigation of epithelial-mesenchymal interactions, tumorigenesis and basement membrane biology in ontogeny and epithelial differentiation.8 Antibodies that react specifically with collagen type X are useful for the study of specific differential tissue expression and the localization of collagen type X.
Immunogène
Application
Forme physique
Autres remarques
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique