P0409
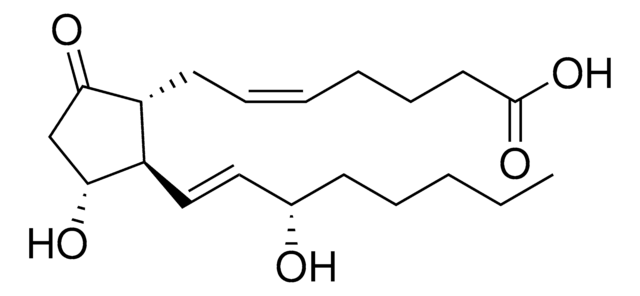
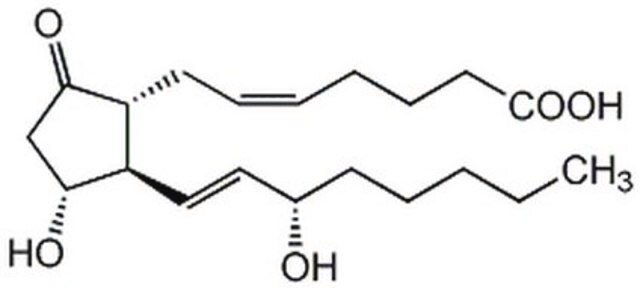
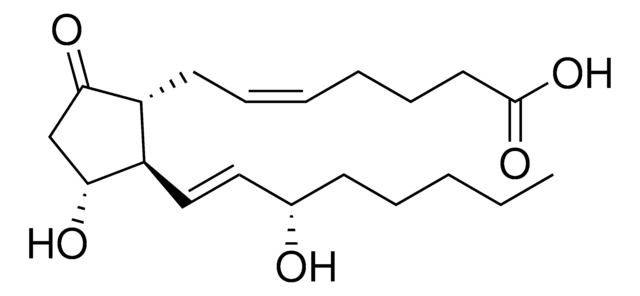
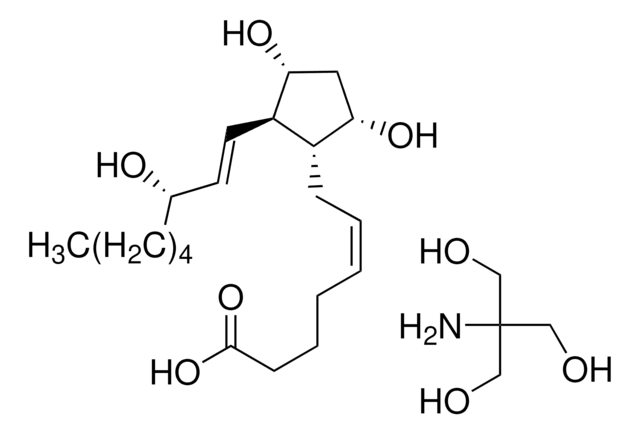
Prostaglandin E2
synthetic, powder, BioReagent, suitable for cell culture
Synonyme(s) :
(5Z,11α,13E,15S)-11,15-Dihydroxy-9-oxoprosta-5,13-dienoic acid, Dinoprostone, PGE2
About This Item
Produits recommandés
Source biologique
synthetic
Niveau de qualité
Gamme de produits
BioReagent
Pureté
≥93% (HPLC)
Forme
powder
Puissance
0.25-100 ng/mL
Technique(s)
cell culture | mammalian: suitable
Solubilité
acetone: 10 mg/mL, clear, colorless to faintly yellow
Conditions d'expédition
ambient
Température de stockage
−20°C
Chaîne SMILES
O[C@@H]1CC([C@H](C/C=C\CCCC(O)=O)[C@H]1/C=C/[C@@H](O)CCCCC)=O
InChI
1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1
Clé InChI
XEYBRNLFEZDVAW-ARSRFYASSA-N
Informations sur le gène
human ... PTGER1(5731) , PTGER2(5732) , PTGER3(5733) , PTGER4(5734) , PTGIR(5739)
mouse ... Ptger1(19216) , Ptger2(19217) , Ptger3(19218) , Ptger4(19219)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Actions biochimiques/physiologiques
Most biologically active prostaglandin. PGE2 induces cervical ripening and parturition; mediates bradykinin-induced vasodilation; regulates adenylyl cyclase. Tumor cells that over-express cyclooxygenase 2 display increased invasiveness, angiogenesis, and resistance to apoptosis that may be due to the PGE2-induced expression of angiogenic factors and stabilization of the anti-apoptotic protein, survivin.
Forme physique
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Repr. 1B
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique