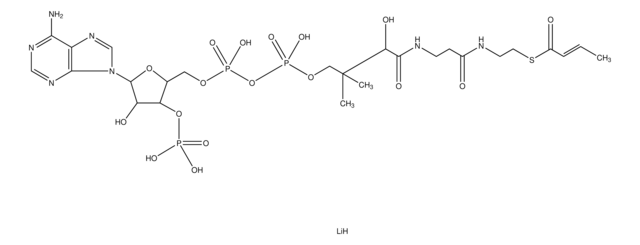
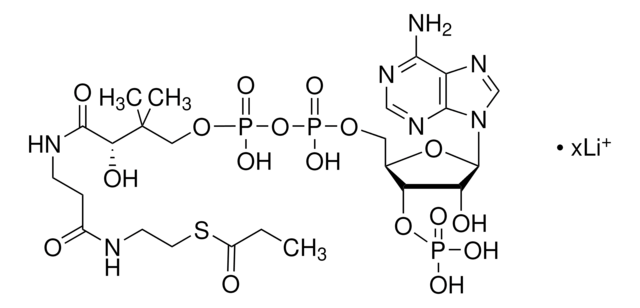
M4414
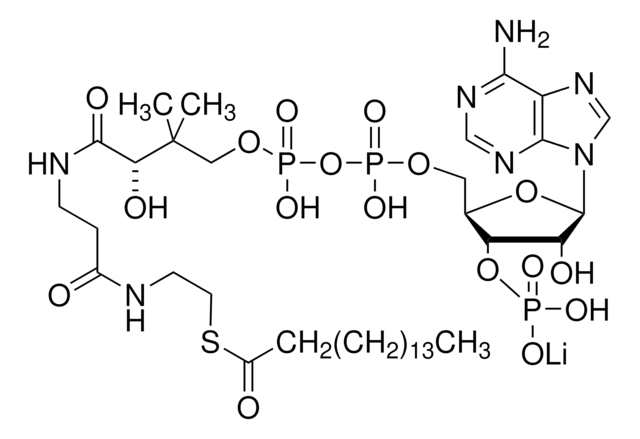
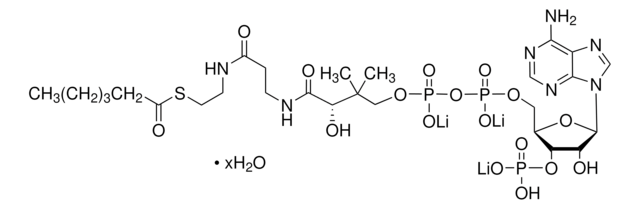
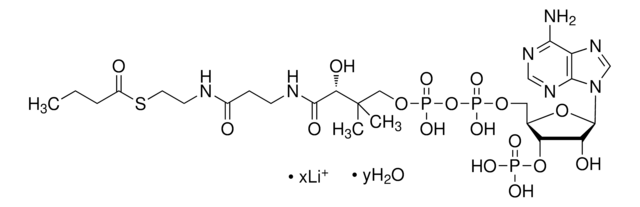
Myristoyl coenzyme A lithium salt
≥80.0% (HPLC), powder, protein myristoylation substrate
Synonyme(s) :
n-Tetradecanoyl Coenzyme A lithium salt
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill) :
C35H62N7O17P3S · xLi+
Numéro CAS:
Poids moléculaire :
977.89 (free acid basis)
Code UNSPSC :
12352202
Nomenclature NACRES :
NA.77
Produits recommandés
Nom du produit
Myristoyl coenzyme A lithium salt, ≥80.0%
Niveau de qualité
Essai
≥80.0%
Température de stockage
−20°C
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Actions biochimiques/physiologiques
Myristoyl coenzyme A is a combination of coenzyme A and myristate. It serves as a substrate in protein myristoylation, catalyzed by the enzyme N-myristoyl transferase. Myristyolation involves the transfer of the myristoyl group to the glycine residue at the amino-terminal of the protein.
Caractéristiques et avantages
This compound is a featured product for Cyclic Nucleotide research. Click here to discover more featured Cyclic Nucleotide products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
Substrats
Long chain fatty acid (C14) covalently linked to Coenzyme A. Substrate for de novo fatty acid synthesis. Fatty acylation has been shown to block G protein-associated calcium release by a direct allosteric modification of a component of the GTP-activated process.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Functional significance of myristoyl moiety in N-myristoyl proteins.
L J Knoll et al.
Methods in enzymology, 250, 405-435 (1995-01-01)
Signal Transduction - Single Cell Techniques, 327-327 (2008)
F Dittrich et al.
European journal of biochemistry, 252(3), 477-485 (1998-04-18)
Elongation of long-chain fatty acids was investigated in yeast mutants lacking endogenous de novo fatty acid synthesis. In this background, in vitro fatty acid elongation was dependent strictly on the substrates malonyl-CoA, NADPH and a medium-chain or long-chain acyl-CoA primer
K E Rys-Sikora et al.
The Journal of biological chemistry, 269(50), 31607-31613 (1994-12-16)
A sensitive and specific GTP-activated Ca2+ translocation process induces rapid Ca2+ movements within cells and appears to reflect G protein-induced membrane fusion or junctional communication between discrete subpopulations of Ca(2+)-pumping organelles (Ghosh, T. K., Mullaney, J. M., Tarazi, F. I.
C M D Swarbrick et al.
Acta crystallographica. Section D, Biological crystallography, 71(Pt 4), 986-995 (2015-04-08)
Acyl-CoA thioesterases catalyse the hydrolysis of the thioester bonds present within a wide range of acyl-CoA substrates, releasing free CoASH and the corresponding fatty-acyl conjugate. The TesB-type thioesterases are members of the TE4 thioesterase family, one of 25 thioesterase enzyme
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique