L8543
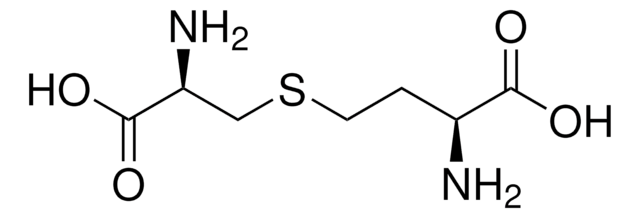
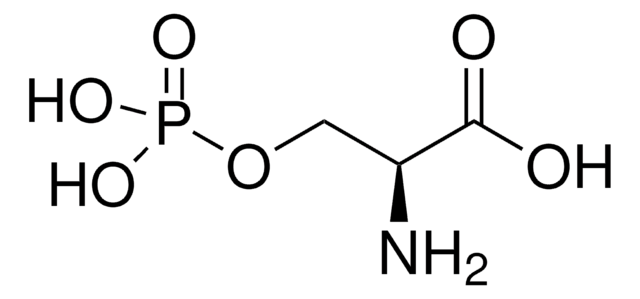
DL-Lanthionine
≥98% (TLC)
Synonyme(s) :
DL-3,3′-thiodi-Alanine, S-(2-amino-2-carboxyethyl)-DL-Cysteine
About This Item
Produits recommandés
Nom du produit
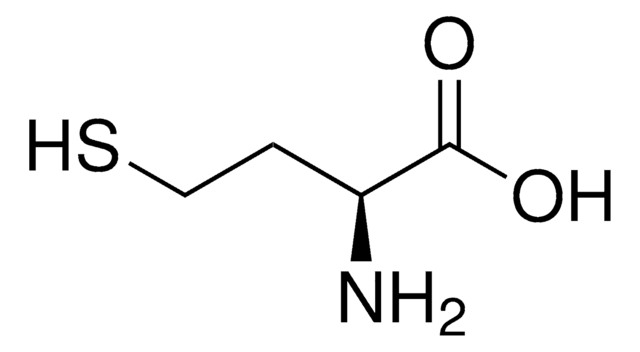
DL-Lanthionine, ≥98% (TLC)
Essai
≥98% (TLC)
Forme
powder
Couleur
white
Solubilité
1 M HCl: soluble
Application(s)
detection
Chaîne SMILES
S(CC(N)C(=O)O)CC(N)C(=O)O
InChI
1S/C6H12N2O4S/c7-3(5(9)10)1-13-2-4(8)6(11)12/h3-4H,1-2,7-8H2,(H,9,10)(H,11,12)
Clé InChI
DWPCPZJAHOETAG-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
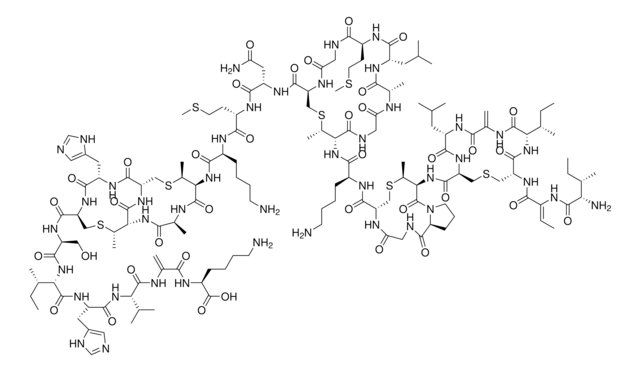
- Synthesis of the lantibiotic lactocin S using peptide cyclizations on solid phase.: This research highlights innovative methods for synthesizing peptide-based antibiotics like lactocin S, utilizing dl-lanthionine to form crucial thioether crosslinks that enhance the activity and stability of these peptides (Ross et al., 2010).
- Cystathionine gamma-lyase of Streptomyces phaeochromogenes.: This research documents the isolation and characterization of an enzyme that processes cystathionine, which involves dl-lanthionine as a structural analog, revealing its role in bacterial physiology and potential applications in biotechnology (Nagasawa et al., 1984).
- The availability of dl-lanthionine for the promotion of growth in young rats when added to a cystine- and methionine-deficient diet.: This historical study explores the nutritional value of dl-lanthionine, investigating its ability to substitute for essential amino acids in growth diets, which helps in understanding its potential use in nutritional supplements (Jones et al., 1948).
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique