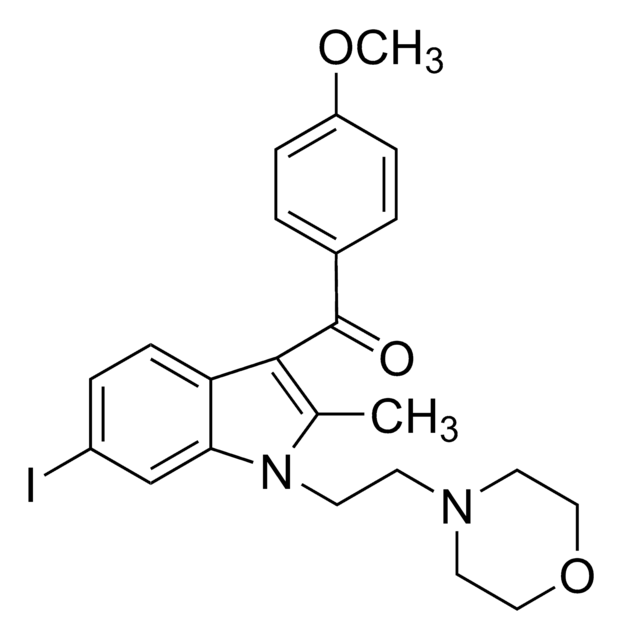
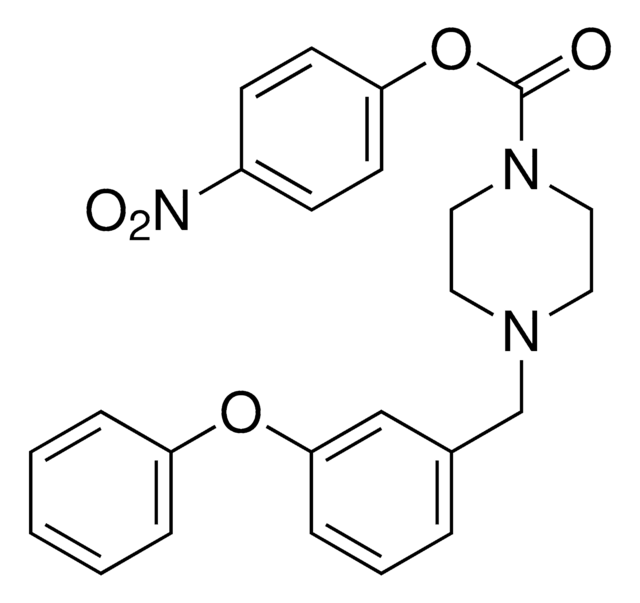
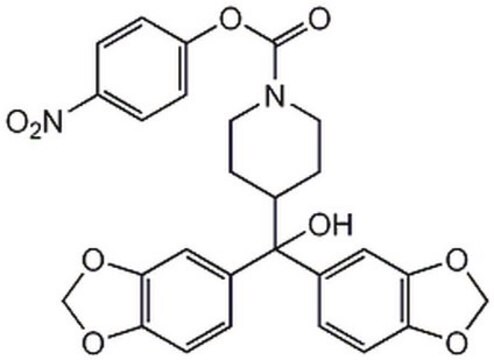
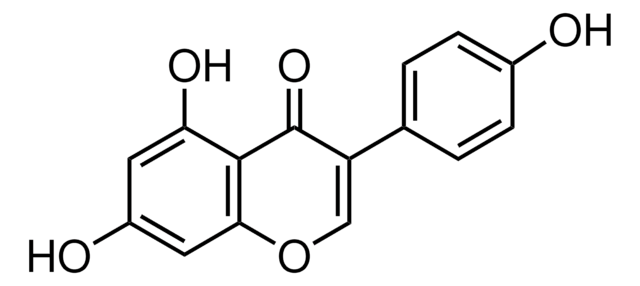
J3455
JZL 184 hydrate
≥97% (HPLC)
Synonyme(s) :
JZL184 hydrate
About This Item
Produits recommandés
Niveau de qualité
Pureté
≥97% (HPLC)
Forme
powder
Couleur
light yellow to yellow-green
Solubilité
DMSO: >20 mg/mL
Température de stockage
2-8°C
Chaîne SMILES
O.OC(C1CCN(CC1)C(=O)Oc2ccc(cc2)[N+]([O-])=O)(c3ccc4OCOc4c3)c5ccc6OCOc6c5
InChI
1S/C27H24N2O9.H2O/c30-26(38-21-5-3-20(4-6-21)29(32)33)28-11-9-17(10-12-28)27(31,18-1-7-22-24(13-18)36-15-34-22)19-2-8-23-25(14-19)37-16-35-23;/h1-8,13-14,17,31H,9-12,15-16H2;1H2
Clé InChI
HNSBVGMOKGQJLT-UHFFFAOYSA-N
Application
Actions biochimiques/physiologiques
Caractéristiques et avantages
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 3 Oral
Code de la classe de stockage
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
Sigma-Aldrich offers many products related to cannabinoid receptors for your research needs.
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique