G0413
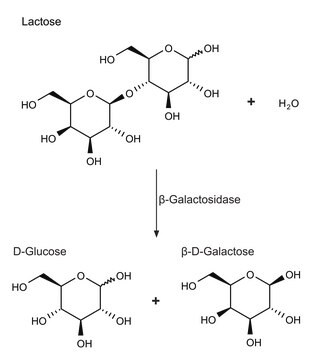
β(1→4)-Galactosidase, positionally specific from Streptococcus pneumoniae
recombinant, expressed in E. coli, buffered aqueous solution
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Numéro CAS:
Numéro MDL:
Code UNSPSC :
12352204
Nomenclature NACRES :
NA.32
Produits recommandés
Produit recombinant
expressed in E. coli
Niveau de qualité
Forme
buffered aqueous solution
Activité spécifique
≥6 units/mg protein
Conditionnement
vial of 0.06 unit
Numéro d'accès UniProt
Conditions d'expédition
wet ice
Température de stockage
2-8°C
Informations sur le gène
human ... GLB1(2720)
Description générale
β-Galactosidase is present in bacteria, fungi, yeast and animal organs. It is also found in fruits, such as apples, almonds and apricots. β-Galactosidase is a tetramer and is made up of four polypeptide chains consisting of amino acids that assemble to form five structural domains. The domains are jelly roll barrel, a central domain that serves as an active site and the remaining domains are composed of β-sandwich and fibronectin.
Application
β(1→4)-Galactosidase, positionally specific from Streptococcus pneumonia has been used:
- as a position-specific enzyme to study its effects in the terminal galactosylation with protective efficacy of glycosphingolipid (GSPL) in mice.
- for the digestion of radioactive oligosaccharides.
- as a position-specific enzymeto study its effects on the virulence profile of avirulent Leishmania donovani clone (A-LD).
Actions biochimiques/physiologiques
β-Galactosidase plays a role in hydrolyzing the D-galactosyl moieties in oligosaccharides, polymers and secondary metabolites. It is widely applicable in the dairy industry to remove lactose from milk and dairy products for the benefit of lactose-intolerant individuals. β-Galactosidase is also applicable in the food industry to improve the sweetness, flavor and solubility.
Définition de l'unité
One unit will hydrolyze 1 μmole of p-nitrophenyl β-D-galactopyranoside per min at pH 5.0 at 37 °C.
Forme physique
Solution in 20 mM Tris-HCl, pH 7.5, 25 mM NaCl
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Virulence attenuation of a UDP-galactose/N-acetylglucosamine beta1, 4 galactosyltransferase expressing Leishmania donovani promastigote
Bhaumik, SK , et al.
Glycoconjugate Journal, 25(5), 459-472 (2008)
Shaima Saqib et al.
3 Biotech, 7(1), 79-79 (2017-05-14)
The enzyme β-galactosidases have been isolated from various sources such as bacteria, fungi, yeast, vegetables, and recombinant sources. This enzyme holds importance due to its wide applications in food industries to manufacture lactose-hydrolyzed products for lactose-intolerant people and the formation
TLR4 and NKT cell synergy in immunotherapy against visceral leishmaniasis
Karmakar S, et al.
PLoS Pathogens, 8(4), 79-79 (2012)
S K Bhaumik et al.
Glycoconjugate journal, 25(5), 459-472 (2008-01-17)
Protozoan parasites of the genus Leishmania are the causative agent of leishmaniasis, a disease whose manifestations in humans range from mild cutaneous lesions to fatal visceral infections. Human visceral leishmaniasis is caused by Leishmania donovani. Long-term culture in vitro leads
beta Galactosidases and their potential applications: a review
Husain Q
Critical Reviews in Biotechnology, 30(1), 41-62 (2010)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique