Description générale
Lot specific orders are not able to be placed through the web. Contact your local sales rep for more details.
Origine de la lignée cellulaire
Muscle
Application
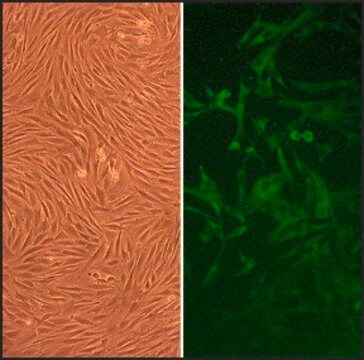
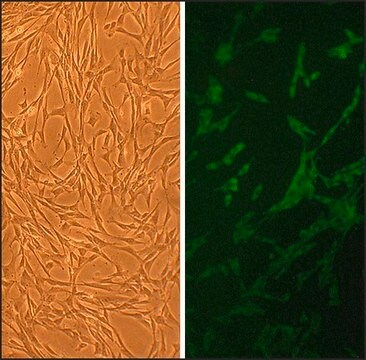
Primary Human Skeletal Muscle Cells (SkMC) are isolated from different skeletal muscles (e.g. Musculus pectoralis major) from adult single donors (lot specific source information is available on request) and are provided in a proliferating format. They are positive for sarcomeric myosin and negative for smooth muscle specific α-actin.New skeletal muscle cells originate from quiescent satellite cells, which are located in the muscle fibers between the basal lamina and the sarcolemma. Quiescent satellite cells are activated by stimuli such as muscle damage. After activation, the cells, now called myoblasts, start to proliferate and fuse with damaged muscle fibers or with one another forming new myotubes.SkMC are optimal for in vitro muscle studies. They proliferate very well in the mitogen-rich PromoCell Skeletal Muscle Cell Growth Medium. Fusion to myotubes with typical multinucleated syncytia can be induced by using the PromoCell Skeletal Muscle Cell Differentiation Medium.
Composants
Mitogen-rich PromoCell Skeletal Muscle Cell Growth Medium
Qualité
Rigid quality control tests are performed for each lot of Human Skeletal Muscle Cells. They are tested for cell morphology, adherence rate, and cell viability. Immunohistochemical tests for cell-type specific markers are carried out for each lot. Growth performance is tested through multiple passages up to 15 population doublings (PD) under culture conditions without antibiotics and antimycotics. Furthermore, the capacity to differentiate into multinucleated syncytia is routinely checked for each lot. In addition, all cells have been tested for the absence of HIV-1, HIV-2, HBV, HCV, HTLV-1, HTLV-2 and microbial contaminants (fungi, bacteria, and mycoplasma).
Avertissement
Although tested negative for HIV-1, HIV-2, HBV, HCV, HTLV-1 and HTLV-2, the cells – like all products of human origin – should be handled as potentially infectious. No test procedure can completely guarantee the absence of infectious agents.
Procédure de repiquage
Click
here for more information.
Autres remarques
Recommended Plating Density: 3500 - 7000 cells per cm2Passage After Thawing: P2Tested Markers: Sarcomeric myosin positiveDifferentiation capacity to multinucleate syncytia testedGuaranteed population doublings: >15
Produits recommandés
Recommended Primary Cell Culture Media:
Link LinkClause de non-responsabilité
Ce produit, destiné à la recherche scientifique, est soumis à une réglementation spécifique en France, y compris pour les activités d′importation et d′exportation (Article L 1211-1 alinéa 2 du Code de la Santé Publique). L′acheteur (c′est-à-dire l′utilisateur FINAL) est tenu d′obtenir une autorisation d′importation auprès du Ministère français de la Recherche, mentionné à l′article L1245-5-1 II du Code de la Santé Publique. En commandant ce produit, vous confirmez détenir l′autorisation d′importation requise.