A9934
Aminopeptidase I from Streptomyces griseus
lyophilized powder, ≥200 units/mg protein
Synonyme(s) :
Leucine Aminopeptidase IV
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Forme
lyophilized powder
Niveau de qualité
Activité spécifique
≥200 units/mg protein
Poids mol.
21 kDa by gel filtration
33 kDa by SDS-PAGE
Composition
Protein, 40-60% Lowry
Température de stockage
−20°C
Description générale
Aminopeptidase I from Streptomyces griseus is a thermostable enzyme with Glu131 and Tyr246 as key active site residues.
Application
Aminopeptidase I from Streptomyces griseus has been used:
- to test the biochar exposure effect on the enzyme activity
- in circular dichroism (CD) spectroscopy studies
- as a positive control in p-nitroanilide degradation assay
Actions biochimiques/physiologiques
Aminopeptidase I from S. griseus has a fairly broad specificity, being able to remove the N-terminal residue of most proteins, except where the penultimate residue is an imino acid. It contains two Zn2+ binding sites. Aminopeptidase I from S. griseus is inhibited by 1,10-phenanthroline and is activated six-fold by Ca2+, which also stabilizes it against heat inactivation. This monomeric zinc metalloprotein has an isoelectric point (pI) of 5.4.
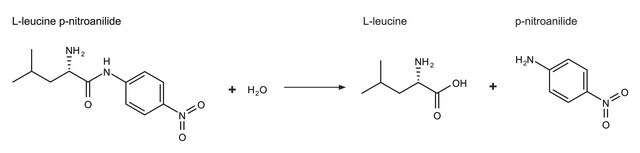
Aminopeptidase I may also be used as a reagent in the assay of endoprotease activities with a synthetic substrate in a two-stage assay. In the first stage, the endoprotease cleaves a peptide, such as Z-Y-X-Leu-p-nitroanilide, with the X, Y, and Z residues being chosen according to the specificity of the endoprotease.
Conditionnement
Package size based on protein content.
Définition de l'unité
One unit will hydrolyze 1.0 μmole of L-leucine-p-nitroanilide to L-leucine and p-nitroaniline per min at pH 8.0, 25 °C and 3.0 mM substrate concentration.
Forme physique
Contains calcium acetate
Notes préparatoires
Reconstitute in 20 mM tricine, pH 8.0, with 0.05% bovine serum albumin. Dilute the enzyme with the reconstitution buffer to 0.15-0.3 U/mL for a working concentration. Solutions should be prepared fresh prior to use.
Autres remarques
Endopeptidase contaminant: Not more than: 0.01 U/mg protein (as μmole tyrosine equivalent per min released from casein.)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
[Autophagy related genes in yeast, S. cerevisiae].
Yoshinori Ohsumi
Tanpakushitsu kakusan koso. Protein, nucleic acid, enzyme, 51(10 Suppl), 1453-1456 (2006-08-23)
Indig, F.E., et al.
Febs Letters, 225, 237-237 (1989)
Taras Y Nazarko et al.
Autophagy, 1(1), 37-45 (2006-07-29)
Yarrowia lipolytica was recently introduced as a new model organism to study peroxisome degradation in yeasts. Transfer of Y. lipolytica cells from oleate/ethylamine to glucose/ammonium chloride medium leads to selective macroautophagy of peroxisomes. To decipher the molecular mechanisms of macropexophagy
A Spungin et al.
European journal of biochemistry, 183(2), 471-477 (1989-08-01)
A heat-stable aminopeptidase with an N-terminal Ala-Pro-Asp-Ile-Pro-Leu sequence has been purified from Streptomyces griseus by heat treatment followed by gel-exclusion and anion-exchange chromatographic procedures. The enzyme is a monomeric zinc metalloenzyme showing an apparent molecular mass of 33 kDa by
Congcong He et al.
Molecular biology of the cell, 19(12), 5506-5516 (2008-10-03)
Autophagy is the degradation of a cell's own components within lysosomes (or the analogous yeast vacuole), and its malfunction contributes to a variety of human diseases. Atg9 is the sole integral membrane protein required in formation of the initial sequestering
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique