PHR1214
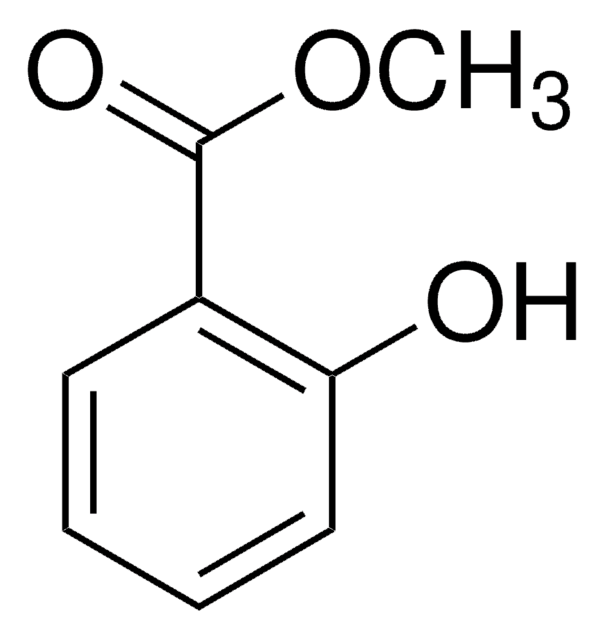
Methyl salicylate
Pharmaceutical Secondary Standard; Certified Reference Material
Synonyme(s) :
Essence de gaulthéria, Ester méthylique d’acide 2-hydroxybenzoïque
About This Item
Produits recommandés
Qualité
certified reference material
pharmaceutical secondary standard
Niveau de qualité
Agence
traceable to USP 1537450
Densité de vapeur
5.26 (vs air)
Pression de vapeur
1 mmHg ( 54 °C)
Famille d'API
methyl salicylate
CofA (certificat d'analyse)
current certificate can be downloaded
Température d'inflammation spontanée
847 °F
Technique(s)
HPLC: suitable
gas chromatography (GC): suitable
Indice de réfraction
n20/D 1.536 (lit.)
pb
222 °C (lit.)
Pf
−8-−7 °C (lit.)
Densité
1.174 g/mL at 25 °C (lit.)
Application(s)
pharmaceutical (small molecule)
Format
neat
Température de stockage
2-8°C
Chaîne SMILES
COC(=O)c1ccccc1O
InChI
1S/C8H8O3/c1-11-8(10)6-4-2-3-5-7(6)9/h2-5,9H,1H3
Clé InChI
OSWPMRLSEDHDFF-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Methyl salicylate is one of the common ingredients present in topical analgesics and rubifacients. It is prescribed for the management of minor muscle aches and pains.
Application
Remarque sur l'analyse
Autres remarques
Note de bas de page
Produits recommandés
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Repr. 2 - Skin Sens. 1B
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
204.8 °F - closed cup
Point d'éclair (°C)
96 °C - closed cup
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique