114472
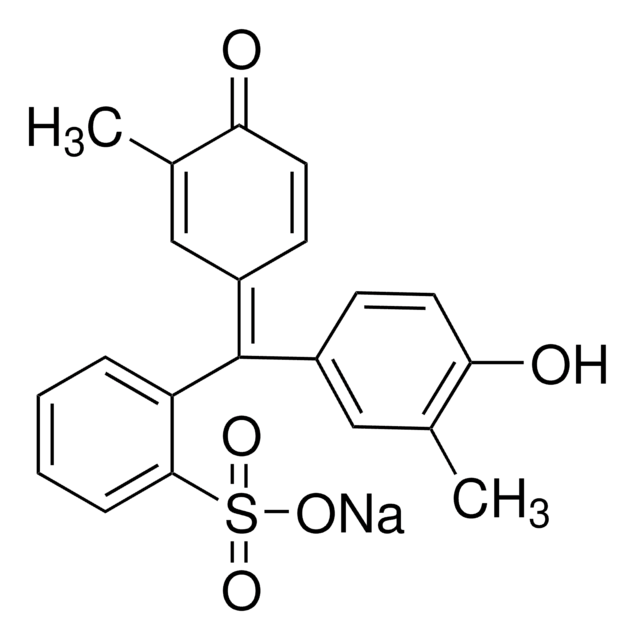
Cresol red
indicator grade, Dye content 95 %
Synonyme(s) :
o-Cresolsulfonphthalein
About This Item
Produits recommandés
Qualité
indicator grade
Niveau de qualité
Forme
powder or crystals
Composition
Dye content, 95%
Technique(s)
titration: suitable
Couleur
red-brown
pH
7.2-8.8, yellow to red
Plage de pH utile
1.8-2.0, orange to yellow(acid)
7.0-8.8, yellow to violet(alkaline)
Pf
290 °C
Densité
0.998 g/cm3
λmax
367 nm (2nd)
570 nm
Application(s)
diagnostic assay manufacturing
hematology
histology
Température de stockage
room temp
Chaîne SMILES
Cc1cc(ccc1O)C2(OS(=O)(=O)c3ccccc23)c4ccc(O)c(C)c4
InChI
1S/C21H18O5S/c1-13-11-15(7-9-18(13)22)21(16-8-10-19(23)14(2)12-16)17-5-3-4-6-20(17)27(24,25)26-21/h3-12,22-23H,1-2H3
Clé InChI
OBRMNDMBJQTZHV-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique