MABN687
Anti-β-amyloid fibril-specific, clone B10, AP Antibody
clone B10, from camel, alkaline phosphatase conjugate
Synonyme(s) :
Amyloid beta A4 protein, ABPP, APPI, APP, Alzheimer disease amyloid protein, Cerebral vascular amyloid peptide, CVAP, PreA4, Protease nexin-II, PN-II, N-APP2.Soluble APP-alpha, S-APP-alpha, Soluble APP-beta, S-APP-beta, C99, Beta-amyloid protein 42, Beta
About This Item
Produits recommandés
Source biologique
camel
Niveau de qualité
Conjugué
alkaline phosphatase conjugate
Forme d'anticorps
purified immunoglobulin
Type de produit anticorps
primary antibodies
Clone
B10, monoclonal
Espèces réactives
human
Technique(s)
ELISA: suitable
dot blot: suitable
immunofluorescence: suitable
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
Isotype
IgG
Numéro d'accès NCBI
Numéro d'accès UniProt
Conditions d'expédition
dry ice
Modification post-traductionnelle de la cible
unmodified
Informations sur le gène
human ... APP(351)
Description générale
Immunogène
Application
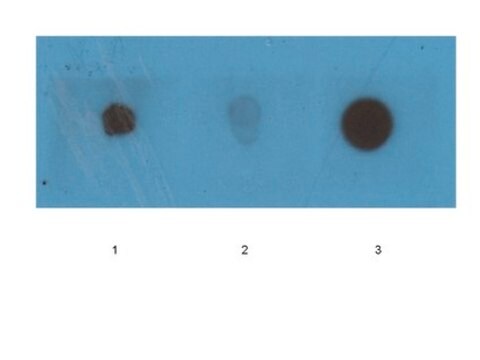
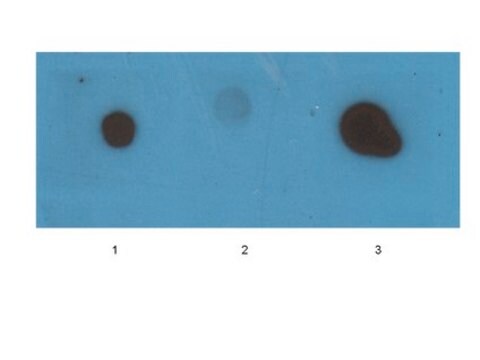
Dot Blot Analysis: A representative lot detected β-amyloid fibril-specific in synthetic Aβ (1–40) peptide (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
Dot Blot Analysis: A representative lot detected β-amyloid fibril-specific in chemically modified fibrils (Haupt, C., et al. (2011). J. Mole. Biol. 405:341-348).
Elisa Analysis: A representative lot detected β-amyloid fibril-specific in N-biotinylated Aβ (1–40) conformers (disaggregated peptide, oligomers, or fibrils) (Morgado, I., et al. (2012). PNAS. 109(31):12503-12508).
Immunohistochemistry Analysis: A representative lot detected β-amyloid fibril-specific in Hippocampal sections from Alzheimer brain tissue (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
Immunoprecipitation Analysis: A representative lot detected β-amyloid fibril-specific in native soluble and dispersible fractions from the brain lysates (Upadhaya, A.R., et al. (2014). BRAIN. 1-17).
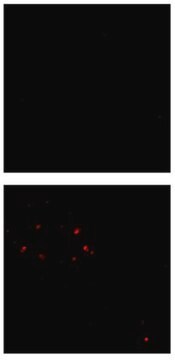
Immunofluorescence Analysis: A representative lot detected β-amyloid fibril-specific in cell culture-derived amyloid plaques (Habicht, G., et al. (2007). PNAS. 104(49):19232-19237).
Qualité
Immunohistochemistry Analysis: A 1:50 dilution of this antibody detected β-amyloid fibril-specific in human Alzheimer′s brain tissue.
Forme physique
Note: This is a Camelid antibody fused to an alkaline phosphatase and does not require a secondary antibody for detection.
Autres remarques
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
nwg
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique