MABN1839
Anti-Amyloid-β (oligomer) Antibody, clone F11G3
clone F11G3, from mouse
Synonyme(s) :
Amyloid beta oligomer, Abeta oligomer, alpha-synuclein oligomer,, alpha-syn oligomer, Prion protein oligomer, PrP oligomer, TAR DNA-binding protein 43 oligomer, TDP-43 oligomer, Tau oligomer
About This Item
Produits recommandés
Source biologique
mouse
Niveau de qualité
Forme d'anticorps
purified immunoglobulin
Type de produit anticorps
primary antibodies
Clone
F11G3, monoclonal
Espèces réactives
all, human, mouse
Technique(s)
ELISA: suitable
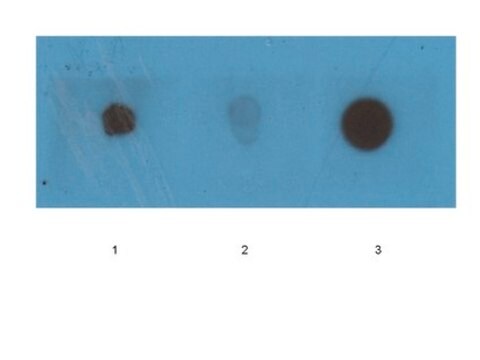
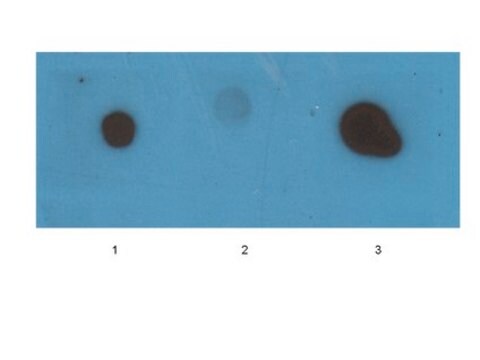
dot blot: suitable
immunofluorescence: suitable
immunoprecipitation (IP): suitable
western blot: suitable
Isotype
IgMκ
Numéro d'accès NCBI
Numéro d'accès UniProt
Conditions d'expédition
dry ice
Modification post-traductionnelle de la cible
unmodified
Informations sur le gène
human ... APP(351) , PRNP(5621) , SNCA(6622) , TARDBP(23435)
Description générale
Spécificité
Immunogène
Application
Western Blotting Analysis: A representative lot detected Ataxin-1 oligomers in soluble cerebella extracts from Atxn1154Q/+, but not wild-type or Atxn-/-, mice (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
Western Blotting Analysis: A representative lot detected cellular beta-sheet oligomer immunoreactivity in HeLa cells transfected with the pathogenic (82Q), but not the non-pathogenic (30Q) form of polyQ Ataxin-1 in transfected Hela cells. Co-transfecting with the native Atxn-1 binding partner Capicua (CIC), but not the binding defective CIC W37A mutant, enhanced the oligomer formation (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
Western Blotting Analysis: A representative lot detected the highest extend of oligomers accumulation in the soluble cerebella extracts among the 28-week old Atxn1154Q/+ mice when compared with samples from 18-week old and 8-week old Atxn1154Q/+ mice, with the 8-week old mice bearing the least oligomer buildup (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
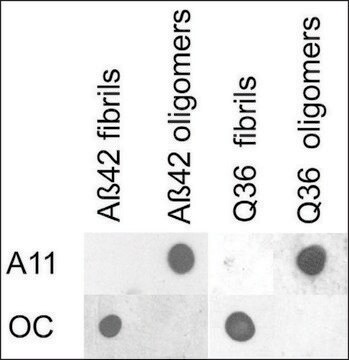
Western Blotting Analysis: A representative lot specifically detected oligomeric, but not monomeric or fibrillar, forms of Aβ42, α-Syn, PrP, and TDP-43 (Guerrero-Muñoz, M.J., et al. (2014). Neurobiol. Dis.71:14-23).
App3/DB/ A representative lot specifically detected oligomeric, but not monomeric or fibrillar, forms of Aβ42, α-Syn, PrP, and TDP-43 (Guerrero-Muñoz, M.J., et al. (2014). Neurobiol. Dis.71:14-23).
ELISA Analysis: A representative lot detected in vitro Aβ42, α-Syn, PrP, and TDP-43 oligomers formation with or without Aβ42 oligomer seeding (Guerrero-Muñoz, M.J., et al. (2014). Neurobiol. Dis.71:14-23).
Immunofluorescence Analysis: A representative lot detected a positive correlation between the ATXN1 beta-sheet oligomer immunoreactivity and the degeneration progression in calbindin-positive Purkinje cells (PCs) by fluorescent immunohistochemistry using paraffin-embedded cerebellum sections from Atxn1154Q/+ mice (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
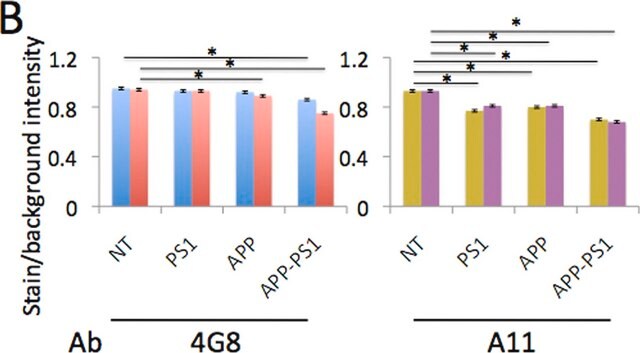
Immunofluorescence Analysis: A representative lot selectively detected beta-sheet oligomer immunoreactivity colocalized with those of Aβ, α-Syn, PrP, and TDP-43 in paraffin-embedded frontal cortex sections from Alzheimer′s diseased brain by fluorescent immunohistochemistry. The beta-sheet oligomer immunoreactivity is not detected in non-AD brains and is distinct from the staining pattern obtained with Thioflavin S (Guerrero-Muñoz, M.J., et al. (2014). Neurobiol. Dis.71:14-23).
Immunoprecipitation Analysis: A representative lot immunoprecipitated Ataxin-1 oligomers from the soluble cerebella extracts of Atxn1154Q/+, but not Atxn-/-, mice (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
Immunocytochemistry Analysis: A representative lot detected cellular beta-sheet oligomer immunoreactivity in HeLa cells transfected with the pathogenic polyQ Ataxin-1 mRFP fusion construct mRFP-ATXN1(82Q) by fluorescent immunocytochemistry. Co-transfecting with the N-terminal fragment of the Atxn-1 binding partner Capicua (CIC), but not the binding defective CIC W37A mutant fragment, enhanced the oligomer formation (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
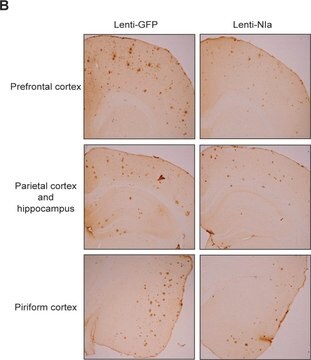
Immunohistochemistry Analysis: A representative lot detected beta-sheet oligomer immunoreactivity in paraffin-embedded cerebellum and cortex sections of Atxn1154Q/+, but not wild-type, mice (Lasagna-Reeves, C.A., et al. (2015). eLlife. 4:e07558).
Neuroscience
Neurodegenerative Diseases
Qualité
Western Blotting Analysis: 2.0 µg/mL of this antibody detected 10 µg of oligomeric amyloid.
Description de la cible
Forme physique
Stockage et stabilité
Handling Recommendations: Upon receipt and prior to removing the cap, centrifuge the vial and gently mix the solution. Aliquot into microcentrifuge tubes and store at -20°C. Avoid repeated freeze/thaw cycles, which may damage IgG and affect product performance.
Autres remarques
Clause de non-responsabilité
Vous ne trouvez pas le bon produit ?
Essayez notre Outil de sélection de produits.
En option
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique