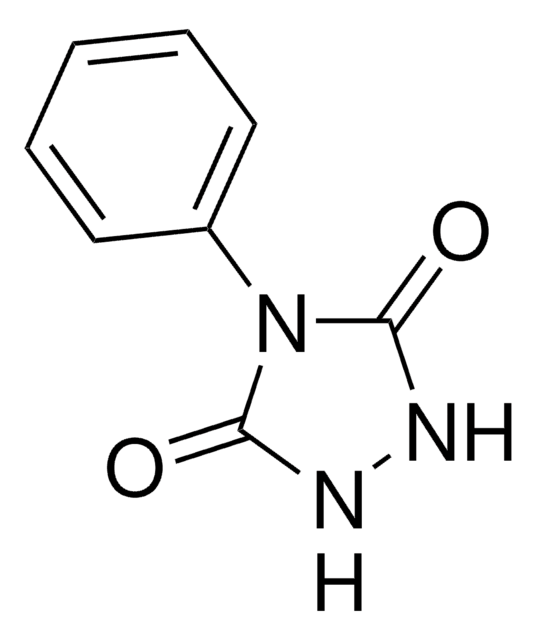
T511552
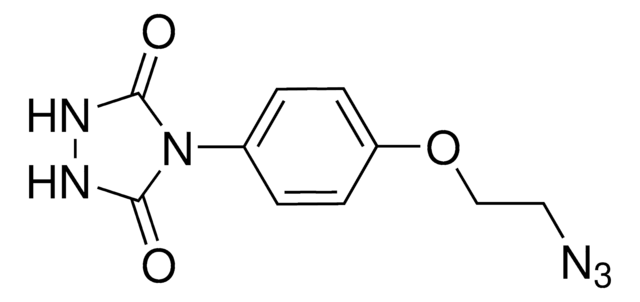
PTAD-Azide
AldrichCPR
Synonyme(s) :
4-(4-(2-Azidoethoxy)phenyl)-1,2,4-triazolidine-3,5-dione, N3-Ph-Ur for e-Y-CLICK
About This Item
Produits recommandés
Chaîne SMILES
O=C(NNC1=O)N1C2=CC=C(OCCN=[N+]=[N-])C=C2
InChI
1S/C10H10N6O3/c11-15-12-5-6-19-8-3-1-7(2-4-8)16-9(17)13-14-10(16)18/h1-4H,5-6H2,(H,13,17)(H,14,18)
Clé InChI
MHGMHPVYCVQIET-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Application
Note: PTAD-Azide must be first activated by stirring in a 1:0.98 molar ratio with 1,3-dibromo-5,5-dimethylhydantoin (product # 157902). Activation is evident upon solution color change from colorless to deep red and the activated reagent should be used immediately.
General Procedure for Protein Modification with PTAD.
Part 1: PTAD activation
- Mix together 1:0.98 molar equivalents of unactivated PTAD to 1,3-dibromo-5,5-dimethylhydantoin (product # 157902) in organic solvent (preferred solvents are DMF or acetonitrile; avoid using DMSO)
- Color change is observed from colorless/pale yellow to deep red (approximately 5 min of mixing).
- After the solution turns red, store the now activated reagent on ice and use for protein modification within 30 min.
Part 2: Protein modification
- Add protein solution in mixed phosphate/Tris buffer or Tris buffer (pH should be 6 - 9) to the eppendorf tube (or other vial) containing the activated PTAD reagent prepared above and mix gently at room temperature for up to 30 min. Preferably use 10-fold molar excess of reagent relative to protein. Use protein at a minimum concentration of 1 mg/ml (higher concentrations are preferred for enhanced labeling).
- Remove excess unreacted PTAD by gel filtration.
Conditionnement
Autres remarques
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique