A93607
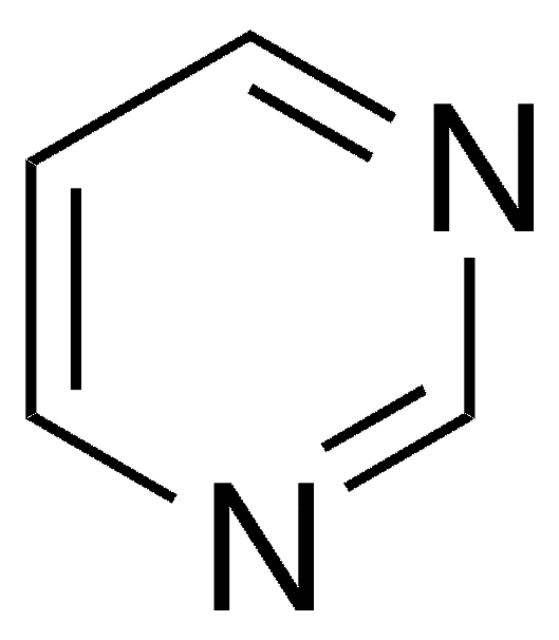
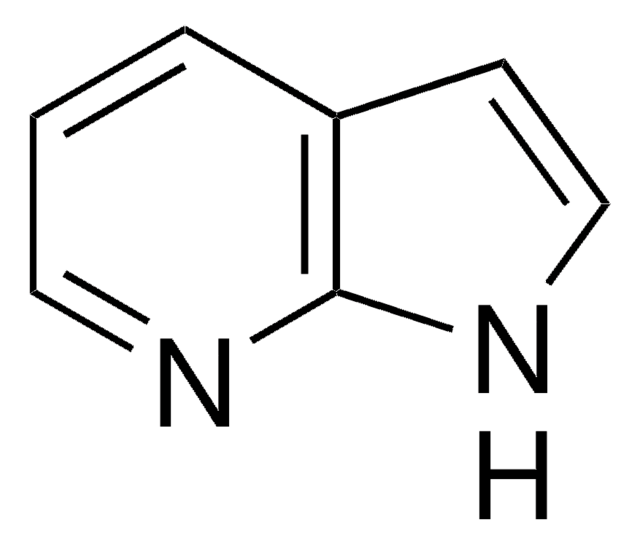
4-Azabenzimidazole
99%
Synonyme(s) :
1H-Imidazo[4,5-b]pyridine
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C6H5N3
Numéro CAS:
Poids moléculaire :
119.12
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
eCl@ss :
32151902
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Pureté
99%
Forme
powder
Pf
148-151 °C (lit.)
Chaîne SMILES
c1cnc2nc[nH]c2c1
InChI
1S/C6H5N3/c1-2-5-6(7-3-1)9-4-8-5/h1-4H,(H,7,8,9)
Clé InChI
GAMYYCRTACQSBR-UHFFFAOYSA-N
Catégories apparentées
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
L Bukowski et al.
Archiv der Pharmazie, 324(2), 121-127 (1991-02-01)
New derivatives of imidazo[4,5-b]pyridine and 9H-dipyrido-[1,2-a:3',2'-d]imidazole were synthesized. Antibacterial activity against Mycobacterium tuberculosis of selected compounds was determined. These data were combined with the corresponding bioactivity data previously generated for two other series of imidazo[4,5-b]pyridines. Analysis of Quantitative Structure-Activity Relationships
S Piras et al.
Farmaco (Societa chimica italiana : 1989), 48(9), 1249-1259 (1993-09-01)
Twenty compounds possessing benzimidazole, imidazo[4,5-b]pyridine and quinoxaline structure bearing either a substituted arylmethylmercapto- or an arylmethylsulfinyl group in position 2 were prepared in order to evaluate an antiulcer and gastroprotective activity in rat pylorus ligature, in comparison with omeprazole at
Fabiola Zapata et al.
Organic letters, 10(1), 41-44 (2007-12-07)
A new redox, chromogenic, and fluorescent chemosensor molecule based on a deazapurine ring selectively senses aqueous Pb2+ in acetonitrile over other metal ions examined: redox shift (DeltaE1/2 = 0.15 V of the Fe(II)/Fe(III) redox couple), the colorless to orange color
Vassilios Bavetsias et al.
Bioorganic & medicinal chemistry letters, 17(23), 6567-6571 (2007-10-16)
A hit generation and exploration approach led to the discovery of 31 (2-(4-(6-chloro-2-(4-(dimethylamino)phenyl)-3H-imidazo[4,5-b]pyridin-7-yl)piperazin-1-yl)-N-(thiazol-2-yl)acetamide), a potent, novel inhibitor of Aurora-A, Aurora-B and Aurora-C kinases with IC(50) values of 0.042, 0.198 and 0.227microM, respectively. Compound 31 inhibits cell proliferation and has good
Ping Lan et al.
European journal of medicinal chemistry, 46(1), 77-94 (2010-11-26)
3D-QSAR and docking studies were performed on sixty imidazo[4,5-b]pyridine derivatives as Aurora A kinase inhibitors. The CoMFA and CoMSIA models using forthy-eight molecules in the training set, gave r(cv)(2) values of 0.774 and 0.800, r(2) values of 0.975 and 0.977
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique
![Imidazo[1,2-a]pyridine 99%](/deepweb/assets/sigmaaldrich/product/structures/109/863/81ccb63f-07c6-4271-b317-1ba58979d455/640/81ccb63f-07c6-4271-b317-1ba58979d455.png)
![3-Methyl-3H-imidazo[4,5-b]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/309/037/0969ed2b-d1ce-49e9-87c8-ef436d41719a/640/0969ed2b-d1ce-49e9-87c8-ef436d41719a.png)


![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate](/deepweb/assets/sigmaaldrich/product/structures/251/439/7a621e74-bfd1-4a43-833c-09adfcc1e0b3/640/7a621e74-bfd1-4a43-833c-09adfcc1e0b3.png)