790389
Difluoro{2-[1-(3,5-dimethyl-2H-pyrrol-2-ylidene-N)ethyl]-3,5-dimethyl-1H-pyrrolato-N}boron
99% (HPLC)
Synonyme(s) :
1,3,5,7,8-Pentamethyl-4,4-difluorro-4-bora-3a,4a-diaza-s-indacene, 2-[1-(3,5-Dimethyl-2H-pyrrol-2-ylidene)ethyl]-3,,5-dimethyl-1H-pyrrole, boron complex, 4,4-Difluoro-1,3,5,7,8-pentamethyl-4-bora-3a,4a-diaza-s-indacene, BODIPY 493/503, PM 546, PMPBF2, Pyrromethene 546, [[2-[1-(3,5-Dimethyl-2H-pyrrol-2-ylidene)ethyl]-3,5-dimethyl-1H-pyrrolato-N1,N2]difluoro]boron
About This Item
Produits recommandés
Pureté
99% (HPLC)
Forme
powder
Pf
262-266 °C
Fluorescence
λex 493 nm; λem 504 nm in methanol
Chaîne SMILES
CC(C1=C(C)C=C(C)N1B(F)2F)=C3[N]2=C(C)C=C3C
InChI
1S/C14H17BF2N2/c1-8-6-10(3)18-13(8)12(5)14-9(2)7-11(4)19(14)15(18,16)17/h6-7H,1-5H3
Clé InChI
DRJHPEGNOPSARR-UHFFFAOYSA-N
Catégories apparentées
Description générale
Application
Also used for solid-state dye laser devices and organic solar cells.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique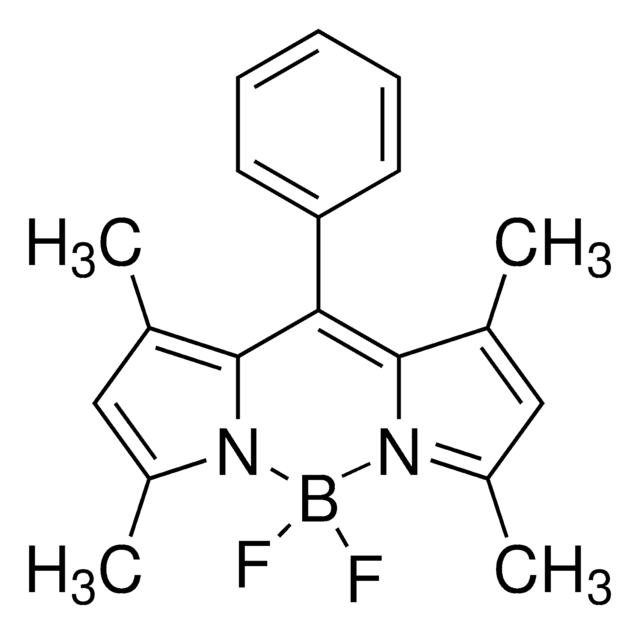
![Difluoro{2-[(3,5-dimethyl-2H-pyrrol-2-ylidene-N)methyl]-3,5-dimethyl-1H-pyrrolato-N}boron 99% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/518/861/c19c64be-654e-472e-a069-30ffccb1a8cd/640/c19c64be-654e-472e-a069-30ffccb1a8cd.png)

![Difluoro(4-(1,1-dimethylethyl)-2-{1-[4-(1,1-dimethylethyl)-3,5-dimethyl-2H-pyrrol-2-ylidene-N]ethyl}-3,5-dimethyl-1H-pyrrol-2-ylidene-N]ethyl}-3,5-dimethyl-1H-pyrrolato-N)boron 98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/207/879/8046aafd-78ca-4fd8-92dc-801de0b6cc53/640/8046aafd-78ca-4fd8-92dc-801de0b6cc53.png)