746746
Lithium hexafluorophosphate solution
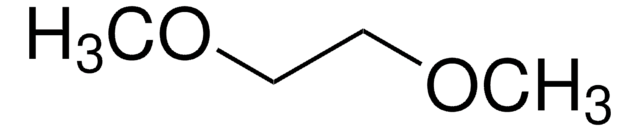
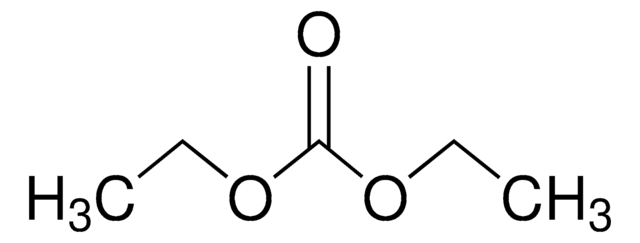
in ethylene carbonate and diethyl carbonate, 1.0 M LiPF6 in EC/DEC=50/50 (v/v), battery grade
Synonyme(s) :
1.0 M LiPF6 EC/DEC=50/50 (v/v)
About This Item
Produits recommandés
Qualité
battery grade
Niveau de qualité
Forme
solution
Caractéristiques du produit alternatif plus écologique
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Concentration
(1.0 M LiPF6 in EC/DEC)
Impuretés
<15 ppm H2O
<50 ppm HF
Couleur
APHA: <50
Point d'ébullition
130 °C
Densité
1.26 g/mL at 25 °C (lit.)
Traces d'anions
chloride (Cl-): ≤1 ppm
sulfate (SO42-): ≤2 ppm
Traces de cations
Ca: ≤1 ppm
Fe: ≤1 ppm
K: ≤1 ppm
Na: ≤1 ppm
Pb: ≤1 ppm
Application(s)
battery manufacturing
Autre catégorie plus écologique
, Enabling
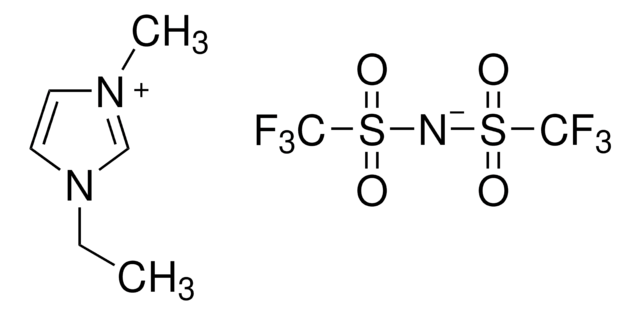
Chaîne SMILES
F[P-](F)(F)(F)(F)F.[Li+]
InChI
1S/F6P.Li/c1-7(2,3,4,5)6;/q-1;+1
Clé InChI
AXPLOJNSKRXQPA-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
The ready-to-use electrolyte solutions are available in different solvent blends and can support a wide variety of lithium ion battery applications. These solutions are high purity and battery grade thus making them also suitable as standards in LIB research. Customized formulations can be made by inter-mixing the electrolyte solutions or by mixing appropriate of additives.
Autres remarques
- Do not use with glass equipment
- All work should be done very quickly under dry air to prevent electrolytes from water uptake and solvent vaporization.
Informations légales
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT RE 1 Inhalation - STOT RE 2 Oral
Organes cibles
Bone,Teeth, Kidney
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
86.0 °F
Point d'éclair (°C)
30 °C
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Dr. Sun reviews the recent advances in solid-state rechargeable batteries and cover the fundamentals of solid electrolytes in solid-state batteries, the theory of ion conduction, and the structures and electrochemical processes of solid-state Li batteries.
Lithium-ion batteries (LIBs) have been widely adopted as the most promising portable energy source in electronic devices because of their high working voltage, high energy density, and good cyclic performance.
The critical technical challenges associated with the commercialization of electric vehicle batteries include cost, performance, abuse tolerance, and lifespan.
Due to the adverse impact of the continued use of fossil fuels on the earth’s environment and climate, researchers have been asked to develop new approaches for producing power using renewable sources like wind and solar energy
Contenu apparenté
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique