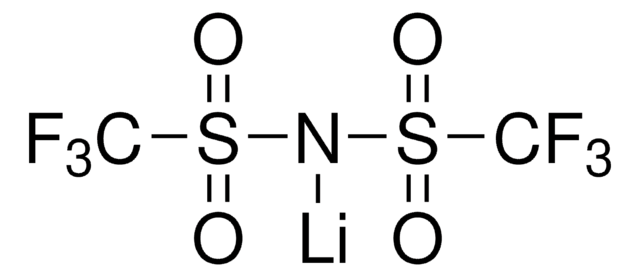
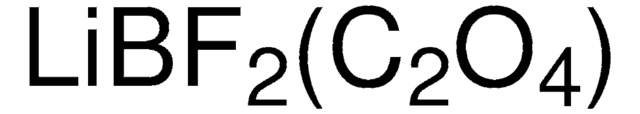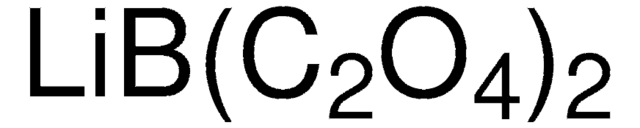450227
Lithium hexafluorophosphate
battery grade, ≥99.99% trace metals basis
Synonyme(s) :
Lithium phosphorus fluoride
About This Item
Produits recommandés
Qualité
battery grade
Niveau de qualité
Pureté
≥99.99% trace metals basis
Forme
powder
Caractéristiques du produit alternatif plus écologique
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impuretés
≤100.0 ppm Trace Metal Analysis
Pf
200 °C (dec.) (lit.)
Application(s)
battery manufacturing
Autre catégorie plus écologique
Chaîne SMILES
[Li+].F[P-](F)(F)(F)(F)F
InChI
1S/F6P.Li/c1-7(2,3,4,5)6;/q-1;+1
Clé InChI
AXPLOJNSKRXQPA-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Autres remarques
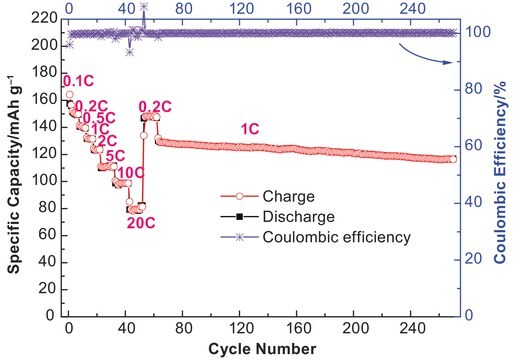
Preparation and characterization of lithium hexafluorophosphate for lithium-ion battery electrolyte.
Produit(s) apparenté(s)
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 3 Oral - Skin Corr. 1A - STOT RE 1 Inhalation
Organes cibles
Bone,Teeth
Code de la classe de stockage
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Increasing fuel costs and concerns about greenhouse gas emissions have spurred the growth in sales of hybrid electric vehicles (HEVs) that carry a battery pack to supplement the performance of the internal combustion engine (ICE).
Increasing fuel costs and concerns about greenhouse gas emissions have spurred the growth in sales of hybrid electric vehicles (HEVs) that carry a battery pack to supplement the performance of the internal combustion engine (ICE).
Dr. Sun reviews the recent advances in solid-state rechargeable batteries and cover the fundamentals of solid electrolytes in solid-state batteries, the theory of ion conduction, and the structures and electrochemical processes of solid-state Li batteries.
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Contenu apparenté
Batteries, fuel cells, and supercapacitors rely on electrochemical energy production. Understand their operation and electron/ion transport separation.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique