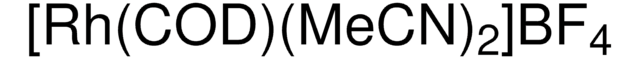695467
1,3,5-Triaza-7-phosphatricyclo[3.3.1.13,7]decane
97%
Synonyme(s) :
1,3,5-Triaza-7-phosphaadamantane, NSC 266642, PTA
About This Item
Produits recommandés
Essai
97%
Forme
solid
Pertinence de la réaction
reagent type: ligand
reaction type: Hydroformylations
reagent type: ligand
reaction type: Hydrogenations
reagent type: ligand
reaction type: Morita-Baylis-Hillman Reactions
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
Pf
244-250 °C
Groupe fonctionnel
phosphine
Chaîne SMILES
C1N2CN3CN1CP(C2)C3
InChI
1S/C6H12N3P/c1-7-2-9-3-8(1)5-10(4-7)6-9/h1-6H2
Clé InChI
FXXRPTKTLVHPAR-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
Application
- The molecular mechanisms of antimetastatic ruthenium compounds explored through DIGE proteomics.: This study examines the antimetastatic properties of ruthenium compounds using DIGE proteomics. The involvement of 1,3,5-Triaza-7-phosphaadamantane in the complexation with ruthenium and its biological effects were analyzed, highlighting its potential in anticancer therapies (Guidi et al., 2013).
- Synthesis, antimicrobial and antiproliferative activity of novel silver(I) tris(pyrazolyl)methanesulfonate and 1,3,5-triaza-7-phosphadamantane complexes.: This research details the synthesis of novel silver complexes containing 1,3,5-Triaza-7-phosphaadamantane, evaluating their antimicrobial and antiproliferative activities, which demonstrates the compound′s utility in biomedical applications (Pettinari et al., 2011).
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
The use of amines and phosphines in nucleophilic catalysis is well precedented; however, arguably one of the severe limitations with respect to exploiting the more nucleophilic, yet less basic, phosphine in this regard is its air sensitivity.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique
![3,7-Diacetyl-1,3,7-triaza-5-phosphabicyclo[3.3.1]nonane 97%](/deepweb/assets/sigmaaldrich/product/structures/198/979/42d0b946-b026-4831-b284-fcb0e91533d9/640/42d0b946-b026-4831-b284-fcb0e91533d9.png)
![1,4-Bis[(phenyl-3-propanesulfonate) phosphine] butane disodium salt](/deepweb/assets/sigmaaldrich/product/structures/322/102/cc3c448f-049a-41a4-93c3-26d70302d06d/640/cc3c448f-049a-41a4-93c3-26d70302d06d.png)
![[(1,3,5,7-Tetramethyl-6-phenyl-2,4,6-trioxa-6-phosphaadamantane)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate](/deepweb/assets/sigmaaldrich/product/structures/324/001/3ffb4bd2-9c6b-451c-80ee-a217f03ca932/640/3ffb4bd2-9c6b-451c-80ee-a217f03ca932.png)
![2,8,9-Triisobutyl-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane 97%](/deepweb/assets/sigmaaldrich/product/structures/750/287/cc77a98e-fa6c-4d81-9f3e-f392770724ac/640/cc77a98e-fa6c-4d81-9f3e-f392770724ac.png)