655422
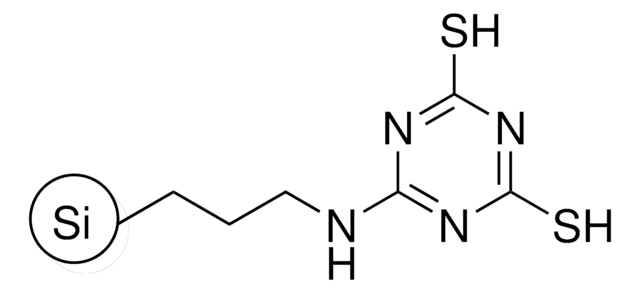
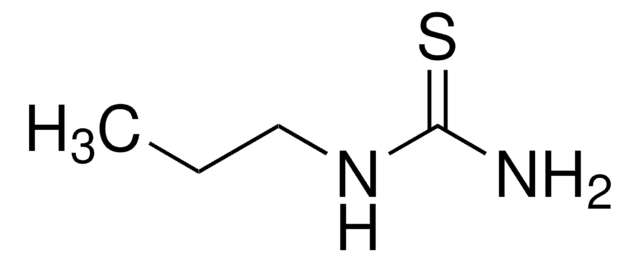
QuadraPure® TU
macroporous, 400-600 μm particle size
Synonyme(s) :
QuadraPure® Thiourea
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Numéro MDL:
Code UNSPSC :
12163800
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pertinence de la réaction
reagent type: catalyst
reagent type: chelator
Taille des particules
400-600 μm
Description générale
QuadraPure® TU is a thiourea-based metal scavenger resin that can be used to prevent metal contamination that occurs during pharmaceutical or fine chemical processing.
Application
Metal Scavenger: Pd, Pt, Ru, Rh, Au, Ag, Cu, Hg, Pb, Cd, Ni, Co, Fe, V, Zn
QuadraPure® TU has been used to remove color from the solution due to leaching of copper species during the copper(I)-mediated 1,2,3-triazole formation via [3+2] cycloaddition of acetylenic compounds with azides. It has also been used as a modifier for carbon pastes to develop QuadraPure® TU residue functionalized resin-modified carbon paste electrode (“TUR-CPE”) for the determination of Pb(II) ions.
Other applications include the removal of metal ions like copper and palladium that leach out during continuous flow coupling reactions.
Other applications include the removal of metal ions like copper and palladium that leach out during continuous flow coupling reactions.
Informations légales
QuadraPure is a registered trademark of Johnson Matthey Finland Oy
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
[3+ 2] Cycloaddition of acetylenes with azides to give 1, 4-disubstituted 1, 2, 3-triazoles in a modular flow reactor.
Smith CD, et al.
Organic & Biomolecular Chemistry, 5(10), 1559-1561 (2007)
Nikzad Nikbin, et al.
Organic Process Research & Development, 11, 458-462 (2007)
Paolo Tosatti et al.
The Journal of organic chemistry, 76(13), 5495-5501 (2011-05-14)
The sequential use of Cu-catalyzed asymmetric allylic alkylation, olefin cross-metathesis, and Ir-catalyzed asymmetric allylic amination allows the concise, stereodivergent synthesis of complex chiral amines with complete regiocontrol and good diastereoselectivity, exemplified by the synthesis of a pair of diastereoisomeric unnatural
Functionalised resin-modified carbon paste sensor for the voltammetric determination of Pb (II) within a wide concentration range.
Mikysek T, et al.
Electrochemical Communications, 10(2), 242-245 (2008)
Michael J. Girgis, et al.
Organic Process Research & Development, 12, 1209-1217 (2008)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique